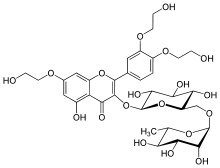
Troxerutin
Troxerutin|
|
| Clinical data |
|---|
| AHFS/Drugs.com |
International Drug Names |
|---|
| ATC code |
C05CA04 (WHO) |
|---|
| Identifiers |
|---|
- 2-[3,4-bis(2-hydroxyethoxy)phenyl]-5-hydroxy-7-(2-hydroxyethoxy)-4-oxo-4H-chromen-3-yl 6-O-(6-deoxy-β-D-mannopyranosyl)-β-D-glucopyranoside
|
| Synonyms |
Hydroxyethylrutoside (HER)
Pherarutin
Trihydroxyethylrutin
3',4',7-Tris[O-(2-hydroxyethyl)]rutin |
|---|
| CAS Number |
7085-55-4  N N |
|---|
| PubChem (CID) |
9896814 |
|---|
| ChemSpider |
4589027  N N |
|---|
| UNII |
7Y4N11PXO8  Y Y |
|---|
| ChEMBL |
CHEMBL3182320  N N |
|---|
| Chemical and physical data |
|---|
| Formula |
C33H42O19 |
|---|
| Molar mass |
742.67518 g/mol |
|---|
| 3D model (Jmol) |
Interactive image |
|---|
C[C@H]1[C@@H]([C@H]([C@H]([C@@H](O1)OC[C@@H]2[C@H]([C@@H]([C@H]([C@@H](O2)Oc3c(=O)c4c(cc(cc4oc3c5ccc(c(c5)OCCO)OCCO)OCCO)O)O)O)O)O)O)O
|
InChI=1S/C33H42O19/c1-14-23(38)26(41)28(43)32(49-14)48-13-21-24(39)27(42)29(44)33(51-21)52-31-25(40)22-17(37)11-16(45-7-4-34)12-20(22)50-30(31)15-2-3-18(46-8-5-35)19(10-15)47-9-6-36/h2-3,10-12,14,21,23-24,26-29,32-39,41-44H,4-9,13H2,1H3/t14-,21+,23-,24+,26+,27-,28+,29+,32+,33-/m0/s1  N NKey:IYVFNTXFRYQLRP-VVSTWUKXSA-N  N N
|
 N N Y (what is this?) (verify) Y (what is this?) (verify) |
|---|
Troxerutin is a flavonol, a type of flavonoid. It is more accurately a hydroxyethylrutoside. It can be isolated from Sophora japonica, the Japanese pagoda tree.
It is used as a vasoprotective.[1]
Troxerutin has been shown in mice to reverse CNS insulin resistance and reduce reactive oxygen species induced by a high-cholesterol diet.[2]
References
- ↑ Riccioni, C.; Sarcinella, R.; Izzo, A.; Palermo, G.; Liguori, M. (2004). "Effectiveness of Troxerutin in association with Pycnogenol in the pharmacological treatment of venous insufficiency". Minerva cardioangiologica. 52 (1): 43–48. PMID 14765037.
- ↑ Lu, J.; Wu, D. -M.; Zheng, Z. -H.; Zheng, Y. -L.; Hu, B.; Zhang, Z. -F. (2011). "Troxerutin protects against high cholesterol-induced cognitive deficits in mice". Brain. 134 (3): 783–797. doi:10.1093/brain/awq376. PMID 21252113.
Flavonols and their conjugates |
|---|
|
| Backbone | |
|---|
|
| Flavonols | Aglycones | |
|---|
| Conjugates | | |
|---|
| |
- Afzelin (Kaempferol 3-rhamnoside)
- Astragalin (kaempferol 3-O-glucoside)
- Kaempferitrin (kaempferol 3,7-dirhamnoside)
- Juglanin (Kaempferol 3-O-arabinoside)
- Kaempferol 3-alpha-L-arabinopyranoside
- Kaempferol 3-alpha-D-arabinopyranoside
- Kaempferol 7-alpha-L-arabinoside
- Kaempferol 7-O-glucoside
- Kaempferol 3-lathyroside
- Kaempferol 4'-rhamnoside
- Kaempferol 5-rhamnoside
- Kaempferol 7-rhamnoside
- Kaempferol 7-O-alpha-L-rhamnofuranoside
- Kaempferol 3-xyloside
- Kaempferol 7-xyloside
- Robinin (kaempferol-3-O-robinoside-7-O-rhamnoside)
- Kaempferol 3-O-rutinoside
- Sophoraflavonoloside (Kaempferol 3-O-sophoroside)
- Trifolin (Kaempferol 3-O-beta-D-galactoside)
|
|---|
| | |
|---|
| | |
|---|
|
|---|
|
|---|
|
| O-Methylated flavonols | Aglycones | |
|---|
| Glycosides | of isorhamnetin |
- Narcissin (Isorhamnetin 3-O-rutinoside)
- Isorhamnetin 3-O-glucoside
- Tamarixetin 7-rutinoside
|
|---|
| other |
- Azalein (Azaleatin 3-O-α-L-rhamnoside)
- Centaurein (Centaureidin 7-O-glucoside)
- Eupalin (Eupalitin 3-0-rhamnoside)
- Eupatolin (Eupatolitin 3-O-rhamnoside)
- Jacein (Jaceidin 7-O-glucoside)
- Patulitrin (Patuletin 7-O-glucoside
- Xanthorhamnin (Rhamnetin glycoside)
|
|---|
|
|---|
|
|---|
|
| Derivative flavonols | Aglycones |
- Noricaritin
- Dihydronoricaritin
|
|---|
| Glycosides | |
|---|
|
|---|
|
| Pyranoflavonols | |
|---|
|
| Furanoflavonols | |
|---|
|
| Semisynthetic | |
|---|