ZM-241,385
 | |
| Names | |
|---|---|
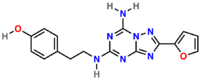
| IUPAC name
4-(2-(7-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-5-ylamino)ethyl)phenol | |
| Identifiers | |
| 139180-30-6 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL113142 |
| ChemSpider | 153646 |
| ECHA InfoCard | 100.216.533 |
| 405 | |
| PubChem | 176407 |
| |
| |
| Properties | |
| C16H15N7O2 | |
| Molar mass | 337.34 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
ZM-241,385 is a high affinity antagonist ligand selective for the adenosine A2A receptor.[1]
In animal models, ZM-241,385 has been shown to protect against beta amyloid neurotoxicity and therefore may be useful as a treatment for Alzheimer's disease.[2] ZM-241,385 has also been shown to enhance L-DOPA derived dopamine release and therefore may be useful in the treatment of Parkinson's disease.[3]
References
- ↑ Palmer TM, Poucher SM, Jacobson KA, Stiles GL (December 1995). "125I-4-(2-(7-amino-2-(2-furyl)(1,2,4)triazolo(2,3-a)(1,3,5) triazin-5-yl-amino)ethyl)phenol, a high affinity antagonist radioligand selective for the A2a adenosine receptor". Molecular Pharmacology. 48 (6): 970–4. PMC 3479638
 . PMID 8848012.
. PMID 8848012. - ↑ Dall'Igna OP, Porciúncula LO, Souza DO, Cunha RA, Lara DR, Dall'lgna OP (April 2003). "Neuroprotection by caffeine and adenosine A2A receptor blockade of beta-amyloid neurotoxicity". British Journal of Pharmacology. 138 (7): 1207–9. doi:10.1038/sj.bjp.0705185. PMC 1573785
 . PMID 12711619.
. PMID 12711619. - ↑ Gołembiowska K, Dziubina A (September 2004). "Striatal adenosine A(2A) receptor blockade increases extracellular dopamine release following l-DOPA administration in intact and dopamine-denervated rats". Neuropharmacology. 47 (3): 414–26. doi:10.1016/j.neuropharm.2004.04.018. PMID 15275831.
External links
ZM 241385 at the US National Library of Medicine Medical Subject Headings (MeSH)
This article is issued from Wikipedia - version of the 11/23/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.