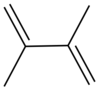
Dimethylbutadiene
 | |
| Names | |
|---|---|
| IUPAC name
2,3-Dimethyl-1,3-butadiene | |
| Other names
Biisopropenyl; Diisopropenyl; 2,3-Dimethylbuta-1,3-diene; 2,3-Dimethylbutadiene; 2,3-Dimethylenebutane | |
| Identifiers | |
| 513-81-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 10124 |
| ECHA InfoCard | 100.007.430 |
| PubChem | 10566 |
| |
| |
| Properties | |
| C6H10 | |
| Molar mass | 82.15 g·mol−1 |
| Density | 0.7222g / cm3[1] |
| Melting point | −76 °C (−105 °F; 197 K) |
| Boiling point | 69 °C (156 °F; 342 K) |
| Vapor pressure | 269 mm Hg (37.7 °C) |
| Hazards | |
| Main hazards | Flammable and irritant |
| GHS pictograms |  |
| EU classification (DSD) |
|
| Flash point | −1 °C (30 °F; 272 K) [2] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dimethylbutadiene, formally referred to as 2,3-dimethyl-1,3-butadiene, is an organic compound with the formula (CH3)2C4H4. It is colorless liquid which served an important role in the early history of synthetic rubber. It is now a specialty reagent.
Synthesis
Dimethylbutadiene is readily prepared by an acid catalyzed dehydration reaction of pinacol:[3]
- 3 C6H14O2 → C6H10 + 2 C6H12O + 4 H2O
The current industrial route involves dimerization of propene followed by dehydrogenation.[4]
Applications
In 1909, Fritz Hofmann and a team working at Bayer succeeded in polymerizing dimethylbutadiene. It was then called methyl isoprene because it has one more methyl group than isoprene. Their polymer was the first synthetic rubber.[5] The polymer had a number of deficiencies relative to natural rubber.[6] The Bayer synthesis of dimethylbutadiene involved the dehydration of pinacol, as described above.[4]
Reactions
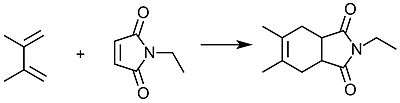
Dimethylbutadiene readily undergoes Diels-Alder reactions and reacts faster than 1,3-butadiene. Its effectiveness in this reaction is attributed to the stabilization of the cis-conformation owing to the influence of the methyl groups on the C2 and C3 positions.
References
- ↑ Haynes, W. M.; Lide, D. R. (2012). CRC Handbook of Chemistry and Physics 93rd Ed. CRC Press/Taylor and Francis. ISBN 1439880492.
- ↑ "CSID:10124". Retrieved 19 October 2012.
- ↑ C. F. H. Allen, Alan Bell, L. W. Newton, and E. R. Coburn (1942). "2,3-Dimethyl-1,3-butadiene". Org. Synth. 22: 39.; Coll. Vol., 3, p. 312
- 1 2 Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke, Hartmut (2000), "Hydrocarbons", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a13_227.
- ↑ The Moving Powers of Rubber, Leverkusen, Germany: LANXESS AG: 20.
- ↑ "A Poor Substitute". Retrieved 18 October 2012.