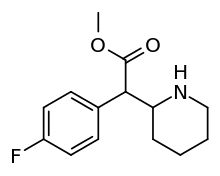
4-Fluoromethylphenidate
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | 1354631-33-6 |
| PubChem (CID) | 70876096 |
| ChemSpider |
26350585 |
| Chemical and physical data | |
| Formula | C14H18FNO2 |
| Molar mass | 251.3 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
4-Fluoromethylphenidate (also known as 4-FMPH and 4F-MPH) is a stimulant drug that acts as a higher efficiency dopamine reuptake inhibitor than the closely related methylphenidate.[1][2][3]
4-Fluoromethylphenidate was studied further along with other analogues of (±)-threo-methylphenidate (TMP) to assess their potential as anti-cocaine medications. 4F-MPH was reported as having an ED50 mg/kg of 0.26 (0.18–0.36) and a relative potency of 3.33 to Methylphenidate.[4]
Another study found that in the threo-isomers of methylphenidate, the meta- and para-substituted compounds with electron-withdrawing substituents tended to have increased binding potency. Compounds containing fluorine, chlorine, bromine and methyl groups were reported to be more potent than methylphenidate. 4F-MPH was reported as having the following values: [3H]WIN 35428 binding of 35.0 ± 3.0 (2) and [3H]dopamine 142 ± 2.0 (2).[5]
Legal Status
4-Fluoromethylphenidate is a Schedule I controlled substance in the US state Alabama.[6]
See also
- 3,4-Dichloromethylphenidate
- 4-Methylmethylphenidate
- Dexmethylphenidate
- HDEP-28
- HDMP-28
- Isopropylphenidate
- Propylphenidate
References
- ↑ Huw M.L. Davies; Darrin W. Hopper; Tore Hansen; Quixu Liu; Steven R. Childers (April 2004). "Synthesis of methylphenidate analogues and their binding affinities at dopamine and serotonin transport sites". Bioorganic & Medicinal Chemistry Letters. 14 (7): 1799–1802. doi:10.1016/j.bmcl.2003.12.097. PMID 15026075.
- ↑ Milind Misra; Qing Shi; Xiaocong Ye; Ewa Gruszecka-Kowalik; Wei Bu; Zhanzhu Liu; Margaret M. Schweri; Howard M. Deutsch; Carol A. Venanzi (October 2010). "Quantitative structure–activity relationship studies of threo-methylphenidate analogs". Bioorganic & Medicinal Chemistry. 18 (20): 7221–7238. doi:10.1016/j.bmc.2010.08.034. PMID 20846865.
- ↑ Satendra Singh (February 2000). "Chemistry, Design, and Structure−Activity Relationship of Cocaine Antagonists". Chemical Reviews. 100 (3): 925–1024. doi:10.1021/cr9700538. PMID 11749256.
- ↑ M. M. Schweri; H. M. Deutsch; A.T. Massey; S. G. Holtzman (May 2002). "Biochemical and Behavioral Characterization of Novel Methylphenidate Analogs". The Journal of Pharmacology and Experimental Therapeutics. 301 (2): 527–535. doi:10.1124/jpet.301.2.527. PMID 11961053.
- ↑ Howard M. Deutsch; Qing Shi; Ewa Gruszecka-Kowalik; Margaret M. Schweri (March 1996). "Synthesis and Pharmacology of Potential Cocaine Antagonists. 2. Structure−Activity Relationship Studies of Aromatic Ring-Substituted Methylphenidate Analogs". Journal of Medicinal Chemistry. 39 (6): 1201–1209. doi:10.1021/jm950697c. PMID 8632426.
- ↑ "Alabama Senate Bill 333 - Controlled substances, Schedule I, additional synthetic controlled substances and analogue substances included in, trafficking in controlled substance analogues, requisite weight increased, Secs. 13A-12-231, 20-2-23 am'd.". March 2014. Retrieved 28 September 2015.