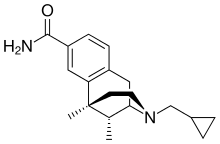
8-Carboxamidocyclazocine
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
911207-68-6 |
| PubChem (CID) | 10086063 |
| ChemSpider | 30845204 |
| Chemical and physical data | |
| Formula | C19H26N2O |
| Molar mass | 298.205 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
8-Carboxamidocyclazocine (8-CAC) is an opioid analgesic drug related to cyclazocine, invented by medicinal chemist Mark P. Wentland and co-workers in Cogswell Laboratory at Rensselaer Polytechnic Institute.[1] Similarly to cyclazocine, 8-CAC acts as an agonist at both the μ and κ opioid receptors, but has a much longer duration of action than cyclazocine, and does not have μ antagonist activity. Unexpectedly it was discovered that the phenolic hydroxyl group of cyclazocine could be replaced by a carboxamido group with only slight loss of potency at opioid receptors, and this discovery has subsequently been used to develop a large number of novel opioid derivatives where the phenolic hydroxy group has been replaced by either carboxamide or a variety of larger groups. Due to their strong κ-opioid agonist activity, these drugs are not suited for use as analgesics in humans, but have instead been researched as potential drugs for the treatment of cocaine addiction.[2][3][4][5][6][7][8][9][10]
See also
References
- ↑ US Patent 6784187 8-carboxamido-2,6-methano-3-benzazocines
- ↑ Wentland, M. P.; Lou, R.; Ye, Y.; Cohen, D. J.; Richardson, G. P.; Bidlack, J. M. (March 2001). "8-Carboxamidocyclazocine analogues: Redefining the structure-activity relationships of 2,6-methano-3-benzazocines". Bioorganic & Medicinal Chemistry Letters. 11 (5): 623–626. doi:10.1016/S0960-894X(01)00014-2. PMID 11266156.
- ↑ Bidlack, J. M.; Cohen, D. J.; McLaughlin, J. P.; Lou, R.; Ye, Y.; Wentland, M. P. (July 2002). "8-Carboxamidocyclazocine: A Long-Acting, Novel Benzomorphan" (pdf). The Journal of Pharmacology and Experimental Therapeutics. 302 (1): 374–380. doi:10.1124/jpet.302.1.374. PMID 12065740.
- ↑ Stevenson, G. W.; Wentland, M. P.; Bidlack, J. M.; Mello, N. K.; Negus, S. S. (December 2004). "Effects of the Mixed-Action κ/μ Opioid Agonist 8-Carboxamidocyclazocine on Cocaine- and Food-Maintained Responding in Rhesus Monkeys". European Journal of Pharmacology. 506 (2): 133–141. doi:10.1016/j.ejphar.2004.10.051. PMID 15588733.
- ↑ Wentland, M. P.; VanAlstine, M.; Kucejko, R.; Lou, R.; Cohen, D. J.; Parkhill, A. L.; Bidlack, J. M. (September 2006). "Redefining the structure-activity relationships of 2,6-methano-3-benzazocines. 4. Opioid receptor binding properties of 8-[N-((4'-phenyl)-phenethyl)carboxamido] analogues of cyclazocine and ethylketocyclazocine". Journal of Medicinal Chemistry. 49 (18): 5635–5639. doi:10.1021/jm060278n. PMID 16942039.
- ↑ VanAlstine, M. A.; Wentland, M. P.; Cohen, D. J.; Bidlack, J. M. (December 2007). "Redefining the structure-activity relationships of 2,6-methano-3-benzazocines. 5. Opioid receptor binding properties of N-((4'-phenyl)-phenethyl) analogues of 8-CAC". Bioorganic & Medicinal Chemistry Letters. 17 (23): 6516–6520. doi:10.1016/j.bmcl.2007.09.082. PMC 2137165
 . PMID 17935988.
. PMID 17935988. - ↑ Wentland, M. P.; Sun, X.; Cohen, D. J.; Bidlack, J. M. (May 2008). "Redefining the structure-activity relationships of 2,6-methano-3-benzazocines. Part 6: Opioid receptor binding properties of cyclic variants of 8-carboxamidocyclazocine". Bioorganic & Medicinal Chemistry. 16 (10): 5653–5664. doi:10.1016/j.bmc.2008.03.066. PMC 2441872
 . PMID 18417347.
. PMID 18417347. - ↑ Wentland, M. P.; Lu, Q.; Ganorkar, R.; Zhang, S. Z.; Jo, S.; Cohen, D. J.; Bidlack, J. M. (January 2009). "Redefining the structure-activity relationships of 2,6-methano-3-benzazocines. Part 7: Syntheses and opioid receptor properties of cyclic variants of cyclazocine". Bioorganic & Medicinal Chemistry Letters. 19 (2): 365–368. doi:10.1016/j.bmcl.2008.11.076. PMID 19091564.
- ↑ Wentland, M. P.; Lou, R.; Lu, Q.; Bu, Y.; Denhardt, C.; Jin, J.; Ganorkar, R.; VanAlstine, M. A.; Guo, C.; Cohen, D. J.; Bidlack, J. M. (April 2009). "Syntheses of novel high affinity ligands for opioid receptors". Bioorganic & Medicinal Chemistry Letters. 19 (8): 2289–2294. doi:10.1016/j.bmcl.2009.02.078. PMC 2791460
 . PMID 19282177.
. PMID 19282177. - ↑ Prisinzano, T. E.; Tidgewell, K.; Harding, W. W. (2005). "k Opioids as Potential Treatments for Stimulant Dependence" (pdf). The AAPS Journal. 7 (3): E592–E599. doi:10.1208/aapsj070361. PMC 2751263
 . PMID 16353938.
. PMID 16353938.