Agkistrodon
| Agkistrodon | |
|---|---|
 | |
| Copperhead, A. contortrix | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Subphylum: | Vertebrata |
| Class: | Reptilia |
| Order: | Squamata |
| Suborder: | Serpentes |
| Family: | Viperidae |
| Subfamily: | Crotalinae |
| Genus: | Agkistrodon Palisot de Beauvois, 1799 |
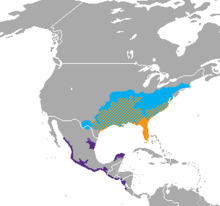 | |
| A. contortrix - cyan A. piscivorus - orange A. bilineatus - violet | |
| Synonyms | |
Agkistrodon is a genus of venomous pit vipers found in North America from the United States south to northern Costa Rica.[1] Three species are currently recognized,[2] all of them polytypic and closely related.[3] Common names include: cottonmouth, copperheads, cantils.[4] Some varieties are known as "moccasins" or "moccasin snakes", such as Agkistrodon piscivorus, the water moccasin.
Name origin
The name Agkistrodon comes from the Greek words agkistron (ἄγκιστρον, 'fishhook', with the irregular transliteration gk rather than the usual nk) and odous (ὁδοὐς, 'tooth,' from the stem ὁδόντ-) and is likely a reference to the fangs.[4]
Some varieties of the genus are given the common name "moccasin" or "moccasin snake" in the United States, which is the Algonquian word for "shoe". The origin of this nickname are unknown, but according to the Word Detective, this may be related to their color and appearance or the silence with which they move.[5] Another source for this name may be the Native American word "mokesoji" of unknown origin and meaning.[6]
Description
Members of this genus have a number of features in common. All species have a relatively broad head with short fangs. A loreal scale is present, except in A. piscivorus. There are usually nine large symmetrical platelike scales on the crown of the head, but in all species these are often irregularly fragmented or have sutures, especially in A. bilineatus. All have a sharply defined canthus rostralis and a vertically elliptical pupil. There are 6-10 (usually 8) supralabial scales and 8-13 (usually 10-11) sublabials. The dorsal scales are mostly keeled and at midbody number 21-25 (usually 23), while A. piscivorus has 23-27 (usually 25). There are 127-157 ventral scales and 36-71 subcaudals. Of the latter, some may be divided. The anal scale is single. All have a color pattern of 10-20 dark crossbands on a lighter ground color, although sometimes the crossbands are staggered as half bands on either side of the body.[4]
The phylogeny of the three species has long been controversial. Studies based on morphological (Gloyd & Conant, 1990) and venom characteristics (Jones, 1976) support the idea that A. bilineatus and A. contortrix are more closely related. However, an analysis of mitochondrial DNA was conducted by Knight et al. (1992), as well as more recent molecular studies (Parkinson et al., 1997, 1999) have concluded that A. bilineatus and A. piscivorus are sister taxa, with A. contortrix being a sister species to them both.[4]
Geographic range
Found in North America from the northeastern and central United States southward through peninsular Florida and southwestern Texas. In Central America on the Atlantic versant from Tamaulipas and Nuevo León southward to the Yucatan Peninsula, Belize and Guatemala. Along the Pacific coastal plane and lower foothills from Sonora south through Guatemala, El Salvador, Honduras and Nicaragua to northwestern Costa Rica.[1]
Behavior
All are semiaquatic to terrestrial and are often found near sources of water. However, A. contortrix and A. bilineatus are also found in dry habitats, often far from permanent streams or ponds.[4]
Reproduction
The members of this genus are all ovoviviparous.[4]
Venom
Pit vipers of the genus Agkistrodon rely on a potent venom they produce for their survival. Used to immobilize prey and fend off predators, one bite can inject enough venom into a human to cause severe pain, swelling, weakness, difficulty breathing, hemorrhaging, gangrene, fever, vomiting, and in rare instances, even death.[7] When concentrations are controlled, however, traditional Chinese medicine has discovered a clinical use for certain species’ venom. Today, this correlation has spread to modern research on the effects of snake venom in cancer patients and victims of strokes (see below).
It is assumed that the venom of all three species is not unlike that of A. contortrix, which contains thrombin-like enzymes that act upon the coagulant activity of the blood. A study of electrophoretic patterns of proteins in venoms among and within populations of A. contortrix and A. piscivorus showed that substantial variation exists (Jones, 1976), and there is no reason to believe that these differences do not correspond with variations in toxicity.[4]
Agkistrodon piscivorus Venom
The largest species of Agkistrodon, Agkistrodon piscivorus, relies on venom to quickly catch prey. In a study conducted at the College of Medicine at the University of Florida, their venom was injected into the lymph fluid of a frog. The frog immediately suffocated because of the collapse of its lung sacs. The venom even resulted in lung constriction when directly applied to the surface of the frog's lungs. To test this, trace amounts of venom were dropped onto a single pulmonary sac in a frog's lung after it was anesthetized and its chest cavity dissected open. A drop of solution containing a venom concentration of 1 mg/mL was enough to cause contraction of the pulmonary artery adventitia after 5-8sec in a frog weighing 40g.[8] The study found, however, that this toxic effect is simply a tool the snake can choose to employ from an accessory venom gland it has. In most instances, the viper injects a venom that tends to immobilize, not kill its prey before ingestion. In this case, the main venom glands secrete a toxin that inhibits the prey’s sympathetic response to flee or fend off its predator. This essentially stuns the animal so that the predator can easily attack.[8]
Deinagkistrodon acutus Venom
One species formerly of Agkistrodon, Deinagkistrodon acutus or the “100 pace snake,” is commonly used for research purposes. Researchers have found that their venom contains protease activity, meaning it attacks and degrades intra- and extracellular proteins.[9] If injected into mice, within 2 hours the venom begins a process known as mesangiolysis (the degeneration and death of cells that line the inner layer of the glomerulus and regulate glomerular filtration in the kidney).[9] Eventually, the kidneys no longer function and the mouse dies.[10]
Venom and Cancer Treatment
Although Agkistrodon venom is lethal to both prey and humans in vivo, when controlled, research shows that the venom has some clinical application. Deinagkistrodon acutus snake venom contains a protein called ACTX-6.[11] This protein was shown to induce apoptosis (cell death) in isolated cancer cells through Fas pathway activation.[11] Fas is a protein that becomes a death receptor in the cellular membrane. When activated, Fas turns on what is called a “Caspase Cascade.”[12] This pathway is made up of a series of proteins called initiator and executioner caspases. Initiator caspases help form an apoptosis initiation factor that eventually activates executioner caspases (see figure 3). Executioner caspases go on to “digest” the cell from the inside out. They cleave cytoskeleton filaments and DNA until the cell completely implodes.[12]
If this pathway can be activated in tumor cells using Agkistrodon venom, then theoretically, the proteins in the venom could be used to target and kill cancerous cells.
Venom and Thrombosis Treatment
Currently, the venom of a different species of pit viper, Agkistrodon rhodostoma, is used to isolate a thrombin-like enzyme called ancrod.[13] This enzyme is used clinically to break down and dissolve thrombi (blood clots) in patients and lower blood viscosity to help prevent heart attack and stroke.[13][14]
Venom and Traditional Chinese Medicine
Deinagkistrodon acutus venom has been used in traditional Chinese medicine for centuries to extract antivenin that is successfully used to treat snakebites.[13][15] Different parts of the snake are also prescribed to help alleviate ailments known as “wind diseases.”[13] Because these snakes move so quickly, it is theorized that substances from their bodies can easily treat these fast moving “wind” syndromes. Agkistrodon acutus is currently used in patients with arthritis, leprosy, tetanus, boils, and, as previously mentioned, tumors.[16] It is believed that the same qualities that make snakes flexible, capable of regenerating skin, and able to inflict paralysis can be transferred to human conditions if applied medicinally.[7] The vipers are prepared by cooking the flesh of the headless body, grinding a paste of snake ash and mixing it with honey, drying the snake and compacting it into a powder, or even injecting their venom intravenously.[7] Although these practices are common in Chinese medicine, there are no current studies that have affirmed the effectiveness of these treatments. It is unknown whether or not these "cures" simply have a placebo effect or actually heal the patients.
Species
| Species[2] | Taxon author[2] | Subsp.*[2] | Common name | Geographic range[1][17] |
|---|---|---|---|---|
| A. bilineatus | (Günther, 1863) | 3 | Cantil/Mexican Ground Pitviper | Mexico and Central America. On the Atlantic side it is found in Mexico in Tamaulipas, Nuevo León, possibly northern Veracruz and Chiapas (in the Middle Grijalva Valley). On the Yucatan Peninsula it occurs in Campeche, Yucatán, Quintana Roo and northern Belize. On the Pacific side it is found from southern Sonora in Mexico south through Guatemala, El Salvador, Honduras and Nicaragua to northwestern Costa Rica. On the Pacific side the distribution is almost continuous, while on the Atlantic side it is disjunct. |
| A. contortrixT | (Linnaeus, 1766) | 5 | Copperhead | The United States (Texas, Oklahoma, Kansas, Missouri, Arkansas, Louisiana, Mississippi, Alabama, Georgia, Florida, South Carolina, North Carolina, Tennessee, Kentucky, Virginia, West Virginia, Illinois, Indiana, Ohio, Iowa, Pennsylvania, Maryland, New Jersey, Delaware, New York, Connecticut, Massachusetts), Mexico (Chihuahua, Coahuila). |
| A. piscivorus | (Lacépède, 1789) | 3 | Cottonmouth Water Moccasin |
The eastern United States from extreme southeastern Virginia, south through peninsular Florida and west to Arkansas, southeastern Kansas, eastern and southern Oklahoma and eastern and central Texas. A few records exist from along the Rio Grande in Texas, but these are thought to represent isolated populations that possibly no longer exist. |
Not including the nominate subspecies. T) Type species.[1]
Taxonomy
This genus was previously much larger and also included the following genera:[1]
- Calloselasma - Ground pit viper found in Southeast Asia (Malaya).
- Deinagkistrodon - The Hundred-pace viper found mostly in southern China.
- Gloydius - Ground pit vipers found in Asia.
- Hypnale - Hump-nosed vipers found in India and Sri Lanka.
See also
- List of crotaline species and subspecies
- Crotalinae by common name
- Crotalinae by taxonomic synonyms
- Snakebite
References
- 1 2 3 4 5 6 McDiarmid RW, Campbell JA, Touré T. 1999. Snake Species of the World: A Taxonomic and Geographic Reference, vol. 1. Herpetologists' League. 511 pp. ISBN 1-893777-00-6 (series). ISBN 1-893777-01-4 (volume).
- 1 2 3 4 "Agkistrodon". Integrated Taxonomic Information System. Retrieved 2 November 2006.
- ↑ Gloyd HK, Conant R. 1990. Snakes of the Agkistrodon Complex: A Monographic Review. Society for the Study of Amphibians and Reptiles. 614 pp. 52 plates. LCCN 89-50342. ISBN 0-916984-20-6.
- 1 2 3 4 5 6 7 Campbell JA, Lamar WW. 2004. The Venomous Reptiles of the Western Hemisphere. Comstock Publishing Associates, Ithaca and London. 870 pp. 1500 plates. ISBN 0-8014-4141-2.
- ↑ "Moccasin". The Word Detective. January 2014. Retrieved November 21, 2016.
- ↑ Catherine C Hopley (1882). Snakes: curiosities and wonders of serpent life. London, Griffith & Farran; New York, E.P. Dutton & co.
Besides that ‘deadly moccasin’ and frequent ‘black snakes,’ there were ‘whip snakes,’ ‘milk snakes,’ and many others which the negroes would bring home as trophies of their courageous slaughter ; but by no scientific names were they known there. Except this name moccasin or mokesoji, which probably conveyed some especial meaning to the aborigines, few of the Indian vernaculars have been preserved in the United States, as we find them in other parts of America, which latter are treated of in chapters xxii. and xxiii. of this work ; but common English names prevail.
line feed character in|quote=at position 505 (help) - 1 2 3 "Agkistrodon acutus pit vipers." Medical-Explorer.com; accessed April 2010.
- 1 2 Gennaro JF, Hall HP, Casey ER, Hayes WK (September 2007). "Neurotropic effects of venoms and other factors that promote prey acquisition". Journal of Experimental Zoology. Part a, Ecological Genetics and Physiology. 307 (9): 488–99. doi:10.1002/jez.405. PMID 17620305.
- 1 2 Morita, Takashi; Churg, Jacob (1983). "Mesangiolysis". Kidney International. 24 (1): 1–9. doi:10.1038/ki.1983.119. PMID 6353041.
- ↑ Schlondorff D (October 1987). "The glomerular mesangial cell: an expanding role for a specialized pericyte". The FASEB Journal. 1 (4): 272–81. PMID 3308611.
- 1 2 Zhang L, Cui L (September 2007). "A cytotoxin isolated from Deinagkistrodon acutus snake venom induces apoptosis via Fas pathway in A549 cells". Toxicology in Vitro. 21 (6): 1095–103. doi:10.1016/j.tiv.2007.04.008. PMID 17544616.
- 1 2 Weinberg, Robert A. The Biology of Cancer. Garland Science, Taylor and Francis Group.(2007);343-350. ISBN 0-8153-4078-8.
- 1 2 3 4 Chen JH, Liang XX, Qiu PX, Yan GM (May 2001). "Thrombolysis effect with FIIa from Agkistrodon acutus venom in different thrombosis model". Acta Pharmacologica Sinica. 22 (5): 420–2. PMID 11743889.
- ↑ Guangmei Yan, Jiashu Chen, Pengxin Qiu,Hong Shan. "Fibrinolysin of Agkistrodon Acutus Venom and its Usage."
- ↑ Qinghua L, Xiaowei Z, Wei Y, et al. (March 2006). "A catalog for transcripts in the venom gland of the Agkistrodon acutus: identification of the toxins potentially involved in coagulopathy". Biochemical and Biophysical Research Communications. 341 (2): 522–31. doi:10.1016/j.bbrc.2006.01.006. PMID 16438937.
- ↑ Subhuti Dharmananda, Ph.D. The Medicinal Use of Snakes in China. Institute for Traditional Medicine, Portland, Oregon. May 1997
- ↑ Savage, JM. The Amphibians and Reptiles of Costa Rica. The University of Chicago Press, 2002, p. 719. ISBN 0-226-73537-0.
Further reading
- Daudin FM. 1801-1803. Histoire naturelle, générale et particulière des reptiles: ouvrage faisant suit à l'histoire naturelle générale et particulière, composée par Leclerc de Buffon; et rédigée par C.S. Sonnini, miembre de plusieurs sociétés savantes. 8 vols. F. Dufart, Paris (for a discussion of the publication date, see F. Harper, 1940, Amer. Midl. Nat. 23: 693).
- Fischer JG. 1813. Zoognosia tabulis synopticus illustrata, in usum praelectionum Academiae Imperialis medico-chirurgicae Mosquensis edita. 3d ed. vol. 1, pt. 3 (Reptiles, Poissons): 57-117. Nicolai Sergeidis Vsevolozsky, Moscow.
- Fitzinger LJ. 1826. Neue Classification der Reptilien nach ihren natürlichen Verwandtschaften: nebst einer Verwandtschafts-Tafel und einem Verzeichnisse der Reptilien-Sammlung des K. K. Zoologischen Museums zu Wien. J.G. Heubner, Vienna. 66 pp.
- Hubbs B, O'Connor B. 2012. A Guide to the Rattlesnakes and other Venomous Serpents of the United States. Tricolor Books. Tempe, Arizona. 129 pp. ISBN 978-0-9754641-3-7.
- Jones JM. 1976. Variation of venom proteins in Agkistrodon snakes from North America. Copeia 1976(3): 558-562.
- Knight A, Densmore III LD, Real ED. 1992. Molecular systematics of the Agkistrodon complex, p. 49-70 In Campbell JA, Brodie Jr. ED. 1992. Biology of the Pitvipers. Texas: Selva. 467 pp. 17 plates. ISBN 0-9630537-0-1.
- Link HF. 1807. Beschreibung der naturalien-sammlung der Universität zu Rostock. Zweite abtheilung, pp. 51–100. Gebruckt bei Adlers Erben, Rostock.
- Palisot de Beauvois AMFJ. 1799. Memoir on Amphibia. Serpentes. Transactions of the American Philosophical Society 4: 362-381.
- Parkinson CL. 1999. Molecular systematics and biogeographical history of pitvipers as determined by mitochondrial ribosomal DNA sequences. Copeia 1999(3): 576-586.
- Parkinson CL, Moody SM, Ahlquist JE. 1997. Phylogenetic relationships of the "Agkistrodon complex" based on mitochondrial DNS data. P. 63-78 In Thorpe RS, Wüster W, Malhotra A. 1997. Venomous snakes: ecology, evolution and snakebite. Clarendon Press, Oxford.
- Sonnini CS, Latreille PA. 1801. Histoire naturelle des reptiles, avec figures dissinees dápres nature. 4 Vols. Paris (for a discussion of the publication date, see F. Harper, 1940, Amer. Midl. Nat. 23: 692-723).
- Troost G. 1836. On a new genus of serpents, and two new species of the genus Heterodon, inhabiting Tennessee. Ann. Lyc. Nat. Hist., New York, 3: 174-190.
- Wagler JG. 1830. Natürliches System der Amphibien, mit vorangehender Classification der Säugthiere und Vögel. Ein Beitrag zur vergleichenden Zoologie. J.G. Cotta, München. 354 pp.
External links
| Wikimedia Commons has media related to Agkistrodon. |
- Agkistrodon at the Reptarium.cz Reptile Database. Accessed 9 August 2007.