Androstanedione
Not to be confused with androstenedione.
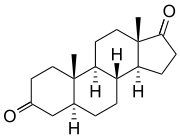 5α-Androstanedione | |
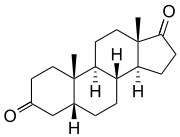 5β-Androstanedione | |
| Names | |
|---|---|
| Systematic IUPAC name
Androstane-3,17-dione | |
| Other names
Dihydroandrostenedione; etiocholane-3,17-dione | |
| Identifiers | |
| 5982-99-0 (α/β) 846-46-8 (α) 1229-12-5 (β) | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:22542 |
| ChemSpider | 2299204 (α/β) |
| PubChem | 3034809 (α/β) |
| |
| |
| Properties | |
| C19H28O2 | |
| Molar mass | 288.43 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Androstanediones are a pair of isomeric steroids which differ at the 5-position. They are distinguished as 5α-androstanedione (5α-androstane-3,17-dione) and 5β-androstanedione (5β-androstane-3,17-dione). In humans, 5α-androstanedione is a metabolic precursor of both testosterone and estrone.[1]
See also
References
- ↑ "Metabocard for Androstanedione". Human Metabolome Database Version 2.5. Human Metabolome Project.
This article is issued from Wikipedia - version of the 11/22/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.