Cobalt(II) sulfate
| | |
 | |
| Names | |
|---|---|
| IUPAC name
Cobalt(II) sulfate | |
| Identifiers | |
| 10124-43-3 13455-64-0 (monohydrate) 10026-24-1 (heptahydrate) | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:53470 |
| ChemSpider | 23338 |
| ECHA InfoCard | 100.030.291 |
| EC Number | 233-334-2 |
| PubChem | 24965 |
| RTECS number | GG3100000 (anhydrous) GG3200000 (heptahydrate) |
| UNII | H7965X29HX |
| |
| |
| Properties | |
| CoSO4(H2O)6 | |
| Molar mass | 154.996 g/mol (anhydrous) 173.01 g/mol (monohydrate) 263.08 g/mol (hexahydrate) 281.103 g/mol (heptahydrate) |
| Appearance | reddish crystalline (anhydrous, monohydrate) pink salt (hexahydrate) |
| Odor | odorless (heptahydrate) |
| Density | 3.71 g/cm3 (anhydrous) 3.075 g/cm3 (monohydrate) 2.019 g/cm3 (hexahydrate) 1.948 g/cm3 (heptahydrate) |
| Melting point | 735 °C (1,355 °F; 1,008 K) |
| anhydrous: 36.2 g/100 mL (20 °C) 38.3 g/100 mL (25 °C) 84 g/100 mL (100 °C) heptahydrate: 60.4 g/100 mL (3 °C) 67 g/100 mL (70 °C) | |
| Solubility | anhydrous: 1.04 g/100 mL (methanol, 18 °C) insoluble in ammonia heptahydrate: 54.5 g/100 mL (methanol, 18 °C) |
| Refractive index (nD) |
1.639 (monohydrate) 1.540 (hexahydrate) 1.483 (heptahydrate) |
| Structure | |
| orthorhombic (anhydrous) monoclinic (monohydrate, heptahydrate) | |
| Hazards | |
| Safety data sheet | JT Baker MSDS |
| EU classification (DSD) |
Carc. Cat. 2 Muta. Cat. 3 Repr. Cat. 2 Toxic (T) Dangerous for the environment (N) |
| R-phrases | R49, R60, R22, R42/43, R68, R50/53 |
| S-phrases | S53, S45, S60, S61 |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
424 mg/kg (oral, rat) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Cobalt(II) sulfate is any of the inorganic compounds with the formula CoSO4(H2O)x. Usually cobalt sulfate refers to the hydrate CoSO4.7H2O, which is one of the most commonly available salts of cobalt.
Properties, preparation, and structure
Cobalt(II) sulfate heptahydrate appears as red monoclinic crystals that liquify around 100 °C and become anhydrous at 250 °C. It is soluble in water, slightly soluble in ethanol, and especially soluble in methanol. The salts are paramagnetic.
It forms by the reaction of metallic cobalt, its oxide, hydroxide, or carbonate with aqueous sulfuric acid.[1]
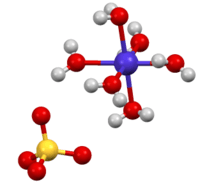
The hexahydrate is a metal aquo complex consisting of octahedral [Co(H2O)6]2+ ions associated with sulfate anions.[2]
Uses
Cobalt is obtained from ores via the sulfate in many cases.[1][3]
Hydrated cobalt(II) sulfate is used in the preparation of pigments, as well as in the manufacture of other cobalt salts. Cobalt pigment is used in porcelains and glass. Cobalt(II) sulfate is used in storage batteries and electroplating baths, sympathetic inks, and as an additive to soils and animal feeds. For these purposes, the cobalt sulfate is produced by treating cobalt oxide with sulfuric acid.[1]
Health issues
Cobalt is essential for most higher forms of life, but more than a few milligrams each day is harmful. Rarely have poisonings resulted from cobalt compounds.[4] Upon inhalation of salts, there is some evidence for carcinogenicity.[1]
References
- 1 2 3 4 John D. Donaldson, Detmar Beyersmann "Cobalt and Cobalt Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a07_281.pub2
- ↑ Elerman, Y. "Refinement of the crystal structure of CoSO4*6H2O" Acta Crystallographica, Section C: Crystal Structure Communications 1988, volume 44, p599-p601. doi:10.1107/S0108270187012447
- ↑ Rarely, cobalt(II) sulfate is found in form of few crystallohydrate minerals, occurring among oxidation zones containing primary Co minerals (like skutterudite or cobaltite). These minerals are: biebierite (heptahydrate), moorhouseite (Co,Ni,Mn)SO4.6H2O, aplowite (Co,Mn,Ni)SO4.4H2O and cobaltkieserite (monohydrate).
- ↑ 11.1.5 The unusual type of myocardiopathy recognized in 1965 and 1966 in Quebec (Canada), Minneapolis (Minnesota), Leuven (Belgium), and Omaha (Nebraska) was associated with episodes of acute heart failure (e/g/, 50 deaths among 112 beer drinkers).
| Salts and esters of the sulfate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2SO4 | He | ||||||||||||||||||
| Li2SO4 | BeSO4 | B | esters ROSO3− (RO)2SO2 |
(NH4)2SO4 N2H6SO4 (NH3OH)2SO4 |
O | F | Ne | ||||||||||||
| Na2SO4 NaHSO4 |
MgSO4 | Al2(SO4)3 Al2SO4(OAc)4 |
Si | P | SO42− | Cl | Ar | ||||||||||||
| K2SO4 KHSO4 |
CaSO4 | Sc2(SO4)3 | Ti(SO4)2 TiOSO4 |
V2(SO4)3 VOSO4 |
CrSO4 Cr2(SO4)3 |
MnSO4 | FeSO4 Fe2(SO4)3 |
CoSO4, Co2(SO4)3 |
NiSO4 | CuSO4 | ZnSO4 | Ga2(SO4)3 | Ge | As | Se | Br | Kr | ||
| RbHSO4 Rb2SO4 |
SrSO4 | Y2(SO4)3 | Zr(SO4)2 | Nb | Mo | Tc | Ru | Rh | PdSO4 | Ag2SO4 | CdSO4 | In2(SO4)3 | SnSO4 | Sb2(SO4)3 | Te | I | Xe | ||
| Cs2SO4 | BaSO4 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2SO4, HgSO4 |
Tl2SO4 | PbSO4 | Bi2(SO4)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La | Ce2(SO4)3 Ce(SO4)2 |
Pr2(SO4)3 | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb2(SO4)3 | Lu | |||||
| Ac | Th | Pa | U(SO4)2 UO2SO4 |
Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
