Doravirine
Not to be confused with drotaverine.
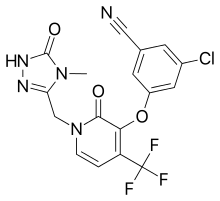 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral[1] |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | MK-1439 |
| CAS Number | 1338225-97-0 |
| PubChem (CID) | 58460047 |
| ChemSpider | 28424197 |
| UNII |
913P6LK81M |
| KEGG | D10624 |
| ChEMBL | CHEMBL2364608 |
| PDB ligand ID | 2KW (PDBe, RCSB PDB) |
| ECHA InfoCard | 100.234.454 |
| Chemical and physical data | |
| Formula | C17H11ClF3N5O3 |
| Molar mass | 425.75 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Doravirine (MK-1439) is a non-nucleoside reverse transcriptase inhibitor under development by Merck & Co. for use in the treatment of HIV/AIDS. Doravirine demonstrated robust antiviral activity and good tolerability in a small clinical study of 7-day monotherapy reported at the 20th Conference on Retroviruses and Opportunistic Infections in March 2013. Doravirine appeared safe and generally well-tolerated with most adverse events being mild-to-moderate.[2][3]
References
- ↑ Collins, Simon; Horn, Tim. "The Antiretroviral Pipeline." (PDF). Pipeline Report. p. 10. Retrieved 6 December 2015.
- ↑ Safety and Antiviral Activity of MK-1439, a Novel NNRTI, in Treatment-naïve HIV+ Patients. Gathe, Joseph et al. 20th Conference on Retroviruses and Opportunistic Infections. 3–6 March 2013. Abstract 100.
- ↑ CROI 2013: MK-1439, a Novel HIV NNRTI, Shows Promise in Early Clinical Trials. Highleyman, Liz. HIVandHepatitis.com. 6 March 2013.
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
