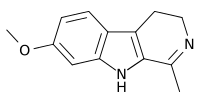
Harmaline
 | |
 | |
| Clinical data | |
|---|---|
| Dependence liability | N/A |
| Routes of administration | Ingestion |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
304-21-2 |
| PubChem (CID) | 5280951 |
| ChemSpider |
10211258 |
| UNII |
CN58I4TOET |
| KEGG |
C06536 |
| ChEBI |
CHEBI:28172 |
| ChEMBL |
CHEMBL340807 |
| ECHA InfoCard | 100.005.594 |
| Chemical and physical data | |
| Formula | C13H14N2O |
| Molar mass | 214.263 g/mol |
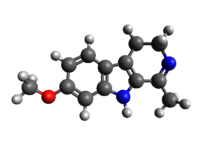
| 3D model (Jmol) | Interactive image |
| Melting point | 232–234 °C (450–453 °F) |
| |
| |
| | |
Harmaline is a fluorescent psychoactive indole alkaloid from the group of harmala alkaloids and beta-carbolines. It is the partially hydrogenated form of harmine.
Occurrence in nature
Various plants contain harmaline including Peganum harmala (Syrian rue) as well as the hallucinogenic beverage ayahuasca, which is traditionally brewed using Banisteriopsis caapi. Present at 3% by dry weight, the harmala alkaloids may be extracted from the Syrian rue seeds.[1]
Effects

Harmaline is a central nervous system stimulant and a "reversible inhibitor of MAO-A (RIMA)".[2] This means that the risk of a hypertensive crisis, a dangerous high blood pressure crisis from eating tyramine-rich foods such as cheese, is likely lower with harmaline than with irreversible MAOIs such as phenelzine.
The harmala alkaloids are psychoactive in humans.[1] Harmaline is shown to act as an acetylcholinesterase inhibitor.[3] Harmaline also stimulates striatal dopamine release in rats at very high dose levels.[4] Since harmaline is a reversible inhibitor of monoamine oxidase A, it could, in theory, induce both serotonin syndrome and hypertensive crises in combination with tyramine, serotonergics, catecholaminergics drugs or prodrugs. Harmaline containing plants and tryptamine containing plants are used in ayahausca brews. The inhibitory effects on monoamine oxidase allows dimethyltryptamine (DMT), the psychologically prominent chemical in the mixture, to bypass the extensive first-pass metabolism it undergoes upon ingestion; allowing a psychologically active quantity of the chemical to exist in the brain for a perceivable period of time.[5] Harmaline forces the anabolic metabolism of serotonin into normelatonin or n-acetylserotonin, and then to melatonin, the body's principle sleep-regulating hormone and a powerful antioxidant.
United States Patent Number 5591738 describes a method for treating various chemical dependencies via the administration of harmaline and or other beta-carbolines.[6]
In a study Harmaline has also been found to induce "vasorelaxant effects" in "isolated rat aorta."[7]
A study found that a single injection of 40 mg/kg in rats or 3 x 25 mg/kg spread over 3 days had visible neurotoxic effects.[8]
Harmaline is known to act as a histamine N-methyltransferase inhibitor.[9] This explains how harmaline elicits its wakefulness-promoting effects.
Legal status
Australia
Harmala alkaloids are considered Schedule 9 prohibited substances under the Poisons Standard (October 2015).[10] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[10]
See also
- Tetrahydroharmine, a similar harmala alkaloid
References
- 1 2 "Syrian Rue". Erowid.
- ↑ Massaro, E. J. (2002). Handbook of Neurotoxicology. Totowa, NJ: Humana Press. p. 237. ISBN 0-89603-796-7.
- ↑ Zheng, X. Y.; Zhang, Z. J.; Chou, G. X.; Wu, T.; Cheng, X. M.; Wang, C. H.; Wang, Z. T. (2009). "Acetylcholinesterase inhibitive activity-guided isolation of two new alkaloids from seeds of Peganum nigellastrum Bunge by an in vitro TLC-bioautographic assay". Archives of Pharmacological Research. 32 (9): 1245–1251. doi:10.1007/s12272-009-1910-x. PMID 19784581.
- ↑ Schwarz, M. J.; Houghton, P. J.; Rose, S.; Jenner, P.; Lees, A. D. (2003). "Activities of Extract and Constituents of Banisteriopsis caapi Relevant to Parkinsonism". Pharmacology Biochemistry and Behavior. 75 (3): 627–633. doi:10.1016/S0091-3057(03)00129-1.
- ↑ "Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions.". NCBI.
- ↑ US patent 5591738, Howard Lotsof, "Method of Treating Chemical Dependency Using β-Carboline Alkaloids, Derivatives and Salts thereof", issued 1997-01-07
- ↑ Berrougui, H.; Martin-Cordero, C.; Khalil, A.; Hmamouchi, M.; Ettaib, A.; Marhuenda, E.; Herrera, M. D. (2006). "Vasorelaxant Effects of Harmine and Harmaline Extracted from Peganum harmala L. Seeds in Isolated Rat Aorta". Pharmacological Research. 54 (2): 150–157. doi:10.1016/j.phrs.2006.04.001. PMID 16750635.
- ↑ O'Hearn, E.; Molliver, M. E. (1993). "Degeneration of Purkinje Cells in Parasagittal Zones of the Cerebellar Vermis after Treatment with Ibogaine or Harmaline". Neuroscience. 55 (2): 303–310. doi:10.1016/0306-4522(93)90500-F. PMID 8377927.
- ↑ Cumming, P; Vincent SR (September 1992). "Inhibition of histamine-N-methyltransferase (HNMT) by fragments of 9-amino-1,2,3,4-tetrahydroacridine (tacrine) and by beta-carbolines". Biochemical Pharmacology. 44 (5): 989–992. doi:10.1016/0006-2952(92)90133-4. PMID 1530666.
- 1 2 Poisons Standard October 2015 https://www.comlaw.gov.au/Details/F2015L01534
Pharmacological Effects of Peganum Harmala L.Root Extract on Isolated Rat small Intestine Shatarat A1, Abuhamdah S2*, Al-Essa M3, Mohammed F4 and Al-Olimat S5
External links
- TIHKAL, #13
- Evans, A. T.; Croft, S. L. (1987). "Antileishmanial Activity of Harmaline and other Tryptamine Derivatives". Phytotherapy Research. 1 (1): 25–27. doi:10.1002/ptr.2650010106.