Hickman line
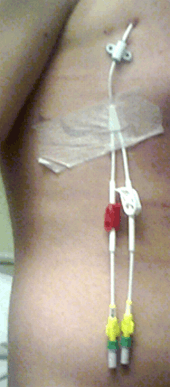
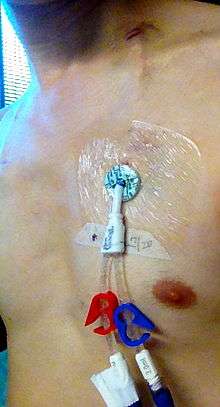
A Hickman line is a central venous catheter most often used for the administration of chemotherapy or other medications, as well as for the withdrawal of blood for analysis. Some types are used mainly for the purpose of apheresis or dialysis. Hickman lines may remain in place for extended periods and are used when long-term intravenous access is required. They are inserted under sedation or a general anesthetic by a radiologist or surgeon. The insertion involves two incisions, one at the jugular vein or another nearby vein or groove, and one on the chest wall. At the former incision site (known as the "entrance" site), a tunnel is created from there through to the latter incision site (known as the "exit" site), and the catheter is pushed through this tunnel until it "exits" the latter incision site. The exit site is where the lumens are seen as coming out of the chest wall. The catheter at the entrance site area is then inserted back through the entrance site and advanced into the superior vena cava, preferably near the junction of it and the right atrium of the heart. The entrance site is sutured. The catheter at the exit site is secured by means of a "cuff" just under the skin at the exit site, and the lumens are held down otherwise by a sterile gauze or dressing centered on the exit site, which also serves the purpose of preventing potential contamination at the exit site. Throughout the procedure, ultrasound and X-rays are used to ascertain the positioning of the catheter.
Long-term venous catheters became available in 1968, and the design was improved by Dr. John W. Broviac (b. 1942), a nephrologist based in East Lansing, et al. in 1973. Hickman et al., after whom the system is named, further modified the principles in 1979 with subcutaneous tunneling and a Dacron cuff that formed an infection barrier. Dr Robert O. Hickman (b. 1927) was a pediatric nephrologist at the Seattle Children's Hospital.
Potential complications of placement of such a line include hemorrhage and pneumothorax during insertion and thrombosis or infection at later stages. Patients with a Hickman line therefore require regular flushes of the catheter with heparin, in order to prevent the line becoming blocked by blood clots. Preventing contamination at the exit site and ensuring that the lumens are flushed frequently is especially important for oncology patients, as they may have become immunocompromised as a result of cytotoxic chemotherapy. Pyrexia (fever) is one of the symptoms of contamination. This symptom and others, including the observance of swelling or bleeding at the exit site, indicate the patient should seek medical attention as soon as possible.
See also
References
- Broviac JW, Cole JJ, Scribner BH (April 1973). "A silicone rubber atrial catheter for prolonged parenteral alimentation". Surg Gynecol Obstet. 136 (4): 602–6. PMID 4632149.
- Hickman RO, Buckner CD, Clift RA, Sanders JE, Stewart P, Thomas ED (June 1979). "A modified right atrial catheter for access to the venous system in marrow transplant recipients". Surg Gynecol Obstet. 148 (6): 871–5. PMID 109934.
- Bard Access Systems, Hickman, Leonard and Broviac Central Venous Catheters instruction manual.
