Isotopes of tennessine
Tennessine (Ts) is the most-recently synthesized synthetic element, and much of the data is hypothetical. As any synthetic element, a standard atomic mass cannot be given. Like all synthetic elements, it has no stable isotopes. The first (and so far only) isotopes to be synthesized were 293Ts and 294Ts in 2009. The longer-lived isotope is 294Ts with a half-life of 51 ms.
Table
| nuclide symbol |
Z(p) | N(n) | isotopic mass (u) |
half-life | decay mode(s) | daughter isotope(s) |
nuclear spin |
|---|---|---|---|---|---|---|---|
| 293Ts | 117 | 176 | 293.20824(89)# | 22 (+8−4) ms[1] | α | 289Uup | |
| 294Ts | 117 | 177 | 294.21046(74)# | 51 (+41−16) ms[2] | α | 290Uup |
Notes
- Values marked # are not purely derived from experimental data, but at least partly from systematic trends.
- Uncertainties are given in concise form in parentheses after the corresponding last digits. Uncertainty values from Ame2003 denote one standard deviation. Values from IUPAC are expanded uncertainties.
Isotopes and nuclear properties
Nucleosynthesis
Target-projectile combinations leading to Z=117 compound nuclei
The below table contains various combinations of targets and projectiles that could be used to form compound nuclei with atomic number 117.
| Target | Projectile | CN | Attempt result |
|---|---|---|---|
| 153Eu | 136Xe | 289Ts | Reaction yet to be attempted |
| 208Pb | 81Br | 289Ts | Reaction yet to be attempted |
| 209Bi | 82Se | 291Ts | Reaction yet to be attempted |
| 232Th | 59Co | 291Ts | Reaction yet to be attempted |
| 231Pa | 58Fe | 289Ts | Reaction yet to be attempted |
| 238U | 55Mn | 293Ts | Reaction yet to be attempted |
| 237Np | 54Cr | 291Ts | Reaction yet to be attempted |
| 244Pu | 51V | 295Ts | Reaction yet to be attempted |
| 243Am | 50Ti | 293Ts | Reaction yet to be attempted |
| 248Cm | 45Sc | 293Ts | Reaction yet to be attempted |
| 250Cm | 45Sc | 295Ts | Reaction yet to be attempted |
| 249Bk | 48Ca | 297Ts | Successful reaction |
| 249Cf | 41K | 290Ts | Reaction yet to be attempted |
| 252Cf | 41K | 293Ts | Reaction yet to be attempted |
| 253Es | 40Ar | 293Ts | Reaction yet to be attempted |
Hot fusion
249Bk (48Ca, xn)297−xTs (x=3,4)
Between July 2009 and February 2010, the team at the JINR (Flerov Laboratory of Nuclear Reactions) ran a 7-month-long experiment to synthesize tennessine using the reaction above.[3] The expected cross-section was of the order of 2 pb. The expected evaporation residues, 293Ts and 294Ts, were predicted to decay via relatively long decay chains as far as isotopes of dubnium or lawrencium.
-
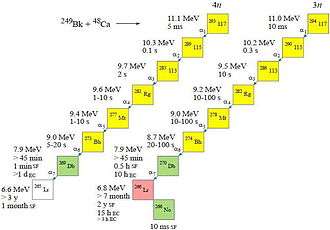
Calculated decay chains from the parent nuclei 293Ts and 294Ts[1]
-

Calculated excitation function for the production of the compound nucleus 297Ts from the reaction249Bk(48Ca,xn)[1]
- ^ a b Roman Sagaidak. "Experiment setting on synthesis of superheavy nuclei in fusion-evaporation reactions. Preparation to synthesis of new element with Z=117" (PDF). Retrieved 2009-07-07.
The team published a scientific paper in April 2010 (first results were presented in January 2010[4]) that six atoms of the neighbouring isotopes 294Ts (one atom) and 293Ts (five atoms) were detected. The heavier isotope decayed by the successive emission of six alpha particles down as far as the new isotope 270Db, which underwent apparent spontaneous fission. On the other hand, the lighter odd-even isotope decayed by the emission of just three alpha particles, as far as 281Rg, which underwent spontaneous fission. The reaction was run at two different excitation energies of 35 MeV (dose 2×1019) and 39 MeV (dose 2.4×1019). Initial decay data was published as a preliminary presentation on the JINR website.[5]
A further experiment in May 2010, looking at the chemistry of one of the decay products, nihonium, identified a further two atoms derived from 294Ts.
Chronology of isotope discovery
| Isotope | Year discovered | Discovery reaction |
|---|---|---|
| 294Ts | 2009 | 249Bk(48Ca,3n) |
| 293Ts | 2009 | 249Bk(48Ca,4n) |
Theoretical calculations
Evaporation residue cross sections
The below table contains various targets-projectile combinations for which calculations have provided estimates for cross section yields from various neutron evaporation channels. The channel with the highest expected yield is given.
DNS = Di-nuclear system; σ = cross section
| Target | Projectile | CN | Channel (product) | σmax | Model | Ref |
|---|---|---|---|---|---|---|
| 209Bi | 82Se | 291Ts | 1n (290Ts) | 15 fb | DNS | [6] |
| 209Bi | 79Se | 288Ts | 1n (287Ts) | 0.2 pb | DNS | [6] |
| 232Th | 59Co | 291Ts | 2n (289Ts) | 0.1 pb | DNS | [6] |
| 238U | 55Mn | 293Ts | 2-3n (291,290Ts) | 70 fb | DNS | [6] |
| 244Pu | 51V | 295Ts | 3n (292Ts) | 0.6 pb | DNS | [6] |
| 248Cm | 45Sc | 293Ts | 4n (289Ts) | 2.9 pb | DNS | [6] |
| 246Cm | 45Sc | 291Ts | 4n (287Ts) | 1 pb | DNS | [6] |
| 249Bk | 48Ca | 297Ts | 3n (294Ts) | 2.1 pb ; 3 pb | DNS | [6][7] |
| 247Bk | 48Ca | 295Ts | 3n (292Ts) | 0.8, 0.9 pb | DNS | [6][7] |
Decay characteristics
Theoretical calculations in a quantum tunneling model with mass estimates from a macroscopic-microscopic model predict the alpha-decay half-lives of isotopes of tennessine (namely, 289–303Ts) to be around 0.1–40 ms.[8][9][10]
References
- ↑ Oganessian, Yu. Ts.; et al. (2013). "Experimental studies of the 249Bk + 48Ca reaction including decay properties and excitation function for isotopes of element 117, and discovery of the new isotope 277Mt". Physical Review C. 87 (5): 054621. Bibcode:2013PhRvC..87e4621O. doi:10.1103/PhysRevC.87.054621.
- ↑ Khuyagbaatar, J.; Yakushev, A.; Düllmann, Ch. E.; et al. (2014). "48Ca+249Bk Fusion Reaction Leading to Element Z=117: Long-Lived α-Decaying 270Db and Discovery of 266Lr". Physical Review Letters. 112 (17): 172501. doi:10.1103/PhysRevLett.112.172501.
- ↑ Tennessine – the 117th element at AtomInfo.ru
- ↑ Recommendations: 31st meeting, PAC for Nuclear Physics
- ↑ Walter Grenier: Recommendations, a PowerPoint presentation at the January 2010 meeting of the PAC for Nuclear Physics
- 1 2 3 4 5 6 7 8 9 Zhao-Qing, Feng; Gen-Ming, Jin; Ming-Hui, Huang; Zai-Guo, Gan; Nan, Wang; Jun-Qing, Li (2007). "Possible Way to Synthesize Superheavy Element Z = 117". Chinese Physics Letters. 24 (9): 2551. arXiv:0708.0159
 . Bibcode:2007ChPhL..24.2551F. doi:10.1088/0256-307X/24/9/024.
. Bibcode:2007ChPhL..24.2551F. doi:10.1088/0256-307X/24/9/024. - 1 2 Feng, Z; Jin, G; Li, J; Scheid, W (2009). "Production of heavy and superheavy nuclei in massive fusion reactions". Nuclear Physics A. 816: 33. arXiv:0803.1117
 . Bibcode:2009NuPhA.816...33F. doi:10.1016/j.nuclphysa.2008.11.003.
. Bibcode:2009NuPhA.816...33F. doi:10.1016/j.nuclphysa.2008.11.003. - ↑ C. Samanta; P. Roy Chowdhury; D. N. Basu (2007). "Predictions of alpha decay half lives of heavy and superheavy elements". Nuclear Physics A. 789: 142. arXiv:nucl-th/0703086
 . Bibcode:2007NuPhA.789..142S. doi:10.1016/j.nuclphysa.2007.04.001.
. Bibcode:2007NuPhA.789..142S. doi:10.1016/j.nuclphysa.2007.04.001. - ↑ P. Roy Chowdhury; C. Samanta; D. N. Basu (2008). "Search for long lived heaviest nuclei beyond the valley of stability". Physical Review C. 77 (4): 044603. arXiv:0802.3837
 . Bibcode:2008PhRvC..77d4603C. doi:10.1103/PhysRevC.77.044603.
. Bibcode:2008PhRvC..77d4603C. doi:10.1103/PhysRevC.77.044603. - ↑ P. Roy Chowdhury; C. Samanta; D. N. Basu (2008). "Nuclear half-lives for α -radioactivity of elements with 100 ≤ Z ≤ 130". Atomic Data and Nuclear Data Tables. 94 (6): 781–806. arXiv:0802.4161
 . Bibcode:2008ADNDT..94..781C. doi:10.1016/j.adt.2008.01.003.
. Bibcode:2008ADNDT..94..781C. doi:10.1016/j.adt.2008.01.003.
External sources
- Isotope masses from:
- M. Wang; G. Audi; A. H. Wapstra; F. G. Kondev; M. MacCormick; X. Xu; et al. (2012). "The AME2012 atomic mass evaluation (II). Tables, graphs and references." (PDF). Chinese Physics C. 36 (12): 1603–2014. Bibcode:2012ChPhC..36....3M. doi:10.1088/1674-1137/36/12/003.
- G. Audi; A. H. Wapstra; C. Thibault; J. Blachot; O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729: 3–128. Bibcode:2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
- Isotopic compositions and standard atomic masses from:
- J. R. de Laeter; J. K. Böhlke; P. De Bièvre; H. Hidaka; H. S. Peiser; K. J. R. Rosman; P. D. P. Taylor (2003). "Atomic weights of the elements. Review 2000 (IUPAC Technical Report)" (PDF). Pure and Applied Chemistry. 75 (6): 683–800. doi:10.1351/pac200375060683.
| Isotopes of livermorium | Isotopes of tennessine | Isotopes of oganesson |
| Table of nuclides | ||
| Isotopes of the chemical elements | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 H |
2 He | ||||||||||||||||
| 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne | ||||||||||
| 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar | ||||||||||
| 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr |
| 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe |
| 55 Cs |
56 Ba |
|
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
| 87 Fr |
88 Ra |
|
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Cn |
113 Nh |
114 Fl |
115 Mc |
116 Lv |
117 Ts |
118 Og |
| |
57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu | ||
| |
89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr | ||
| |||||||||||||||||