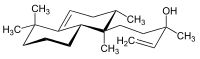
Isotuberculosinol
 | |
| Names | |
|---|---|
| IUPAC name
(3-Methyl-5-[(1R,2S,8aS)-1,2,5,5-tetramethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-1-yl]pent-1-en-3-ol | |
| Other names
Nosyberkol, edaxadiene | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:59685 |
| ChemSpider | 26331708 |
| PubChem | 46224604 |
| |
| |
| Properties | |
| C20H34O | |
| Molar mass | 290.48 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isotuberculosinol, also called nosyberkol or edaxadiene is a diterpene molecule produced by the bacterium Mycobacterium tuberculosis, the causative agent of TB, which aids in its pathogenesis. Isotuberculosinol functions by preventing maturation of the host-cell phagosome in which the bacterium lives.[1][2] Maturation of the phagosome would enable it to kill the bacterium. Mutations in genes involved in the biosynthetic pathway of nosyberkol result in normal development of the phagosome and reduction of mycobacterial infection. These biosynthetic genes include Isotuberculosinol synthase.
Nosyberkol was originally isolated in 2004 from a marine sponge in the ocean near island of Nosy Be after which it was named.[3] The molecule was characterised as a triterpene but the structure was revised following its chemical synthesis in 2010.[4]
References
- ↑ Mann, F. M.; Xu, M.; Chen, X.; Fulton, D. B.; Russell, D. G.; Peters, R. J. (2009). "Edaxadiene: A New Bioactive Diterpene fromMycobacterium tuberculosis". Journal of the American Chemical Society. 131 (48): 17526–17527. doi:10.1021/ja9019287. PMC 2787244
 . PMID 19583202.
. PMID 19583202. - ↑ Mann, F. M.; Peters, R. J. (2012). "Isotuberculosinol: The unusual case of an immunomodulatory diterpenoid from Mycobacterium tuberculosis.". MedChemComm. 3 (8): 899–904. doi:10.1039/c2md20030a. PMC 3733278
 . PMID 23926455.
. PMID 23926455. - ↑ Rudi, A.; Aknin, M.; Gaydou, E.; Kashman, Y. (2004). "Asmarines I, J, and K and Nosyberkol: Four New Compounds from the Marine SpongeRaspailiasp". Journal of Natural Products. 67 (11): 1932–1935. doi:10.1021/np049834b. PMID 15568794.
- ↑ Spangler, J. E.; Carson, C. A.; Sorensen, E. J. (2010). "Synthesis enables a structural revision of the Mycobacterium tuberculosis-produced diterpene, edaxadiene". Chemical Science. 1 (2): 202–205. doi:10.1039/C0SC00284D. PMC 3221386
 . PMID 22114734.
. PMID 22114734.