Lefamulin
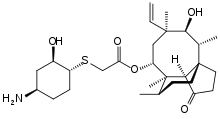 | |
| Clinical data | |
|---|---|
| ATC code | None |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | BC 3781 |
| CAS Number | 1061337-51-6 |
| PubChem (CID) | 25185057 |
| ChemSpider | 32701544 |
| UNII | 21904A5386 |
| ChEMBL | CHEMBL3291398 |
| Chemical and physical data | |
| Formula | C28H45NO5S |
| Molar mass | 507.73 g·mol−1 |
| 3D model (Jmol) | Interactive image |
| |
| |
Lefamulin is a pleuromutilin antibiotic that is being developed by Nabriva Therapeutics for the treatment of acute bacterial skin and skin-structure infections (ABSSSI).[1] It was granted fast track status by the US Food and Drug Administration in 2014. As of May 2016, it is in phase III clinical trials.[2]
Spectrum of activity
Lefamulin has in vitro activity against Streptococcus viridans, Moraxella catarrhalis, Enterococcus faecium, methicillin-resistant Staphylococcus aureus (MRSA), among other bacteria.[2][3]
References
- ↑ Zeitlinger, M; Schwameis, R; Burian, A; Burian, B; Matzneller, P; Müller, M; Wicha, W. W.; Strickmann, D. B.; Prince, W (2016). "Simultaneous assessment of the pharmacokinetics of a pleuromutilin, lefamulin, in plasma, soft tissues and pulmonary epithelial lining fluid". Journal of Antimicrobial Chemotherapy. 71 (4): 1022–6. doi:10.1093/jac/dkv442. PMID 26747098.
- 1 2 H. Spreitzer (23 May 2016). "Neue Wirkstoffe - Lefamulin". Österreichische Apothekerzeitung (in German) (11/2016).
- ↑ Mendes, R. E.; Farrell, D. J.; Flamm, R. K.; Talbot, G. H.; Ivezic-Schoenfeld, Z; Paukner, S; Sader, H. S. (2016). "In vitro activity of lefamulin tested against Streptococcus pneumoniae with defined serotypes, including multidrug-resistant isolates causing lower respiratory tract infections in the United States". Antimicrobial Agents and Chemotherapy: AAC.00627–16. doi:10.1128/AAC.00627-16. PMID 27161634.
This article is issued from Wikipedia - version of the 6/8/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.