Lithium azide
| Names | |
|---|---|
| IUPAC name
lithium azide | |
| Identifiers | |
| 19597-69-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 79536 |
| ECHA InfoCard | 100.039.237 |
| PubChem | 88163 |
| |
| |
| Properties | |
| LiN3 | |
| Molar mass | 48.96 g·mol−1 |
| Melting point | 115 °C (239 °F; 388 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
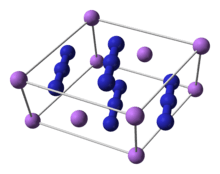
Lithium azide unit cell[1]
Lithium azide is the lithium salt of hydrazoic acid. It is an unstable and toxic compound that decomposes into lithium and nitrogen when heated.
Preparation
It can be prepared by metathesis reaction between sodium azide and lithium nitrate:
- NaN3 + LiNO3 → LiN3 + NaNO3
or lithium sulfate solutions:
- 2 NaN3 + Li2SO4 → 2 LiN3 + Na2SO4[2]
Notes
- ↑ Pringle, G. E.; Noakes, D. E. (February 1968). "The crystal structures of lithium, sodium and strontium azides". Acta Crystallogr. B. 24 (2): 262–269. doi:10.1107/S0567740868002062.
- ↑ http://www.lambdasyn.org/synfiles/lithiumazid.htm
References
- Hofman-Bang, Niels (1957). "Preparation of Lithium Azide". Acta Chemica Scandinavica. 11: 581. doi:10.3891/acta.chem.scand.11-0581.
- Younk, Edward H.; Kunz, A. Barry (1997). "An ab initio investigation of the electronic structure of lithium azide (LiN3), sodium azide (NaN3), and lead azide [Pb(N3)2]". International Journal of Quantum Chemistry. 63 (3): 615. doi:10.1002/(SICI)1097-461X(1997)63:3<615::AID-QUA2>3.0.CO;2-Z.
- Gordienko, A.B.; Poplavnoi, A.S. (1997). "The Application of ab-initio Linear Combination of Pseudo-Atomic-Orbital Scheme for the Electronic Structure of Lithium Azide LiN3". Physica status solidi (b). 202 (2): 941. doi:10.1002/1521-3951(199708)202:2<941::AID-PSSB941>3.0.CO;2-K.
- Zhu, W; Xiao, J; Xiao, H (2006). "Density functional theory study of the structural and optical properties of lithium azide". Chemical Physics Letters. 422: 117. doi:10.1016/j.cplett.2006.02.017.
This article is issued from Wikipedia - version of the 6/17/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.