Lithium borohydride
| Names | |
|---|---|
| IUPAC name
Lithium tetrahydridoborate(1–) | |
| Other names
Lithium hydroborate, Lithium tetrahydroborate Borate(1-), tetrahydro-, lithium, lithium boranate | |
| Identifiers | |
| 16949-15-8 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 55732 |
| ECHA InfoCard | 100.037.277 |
| PubChem | 4148881 |
| RTECS number | ED2725000 |
| |
| |
| Properties | |
| LiBH4 | |
| Molar mass | 21.784 g/mol |
| Appearance | White solid |
| Density | 0.666 g/cm3[1] |
| Melting point | 268 °C (514 °F; 541 K) |
| Boiling point | 380 °C (716 °F; 653 K) decomposes |
| reacts | |
| Solubility in ether | 2.5 g/100 mL |
| Thermochemistry | |
| 82.6 J/mol K | |
| Std molar entropy (S |
75.7 J/mol K |
| Std enthalpy of formation (ΔfH |
-198.83 kJ/mol |
| Hazards | |
| > 180 °C (356 °F; 453 K) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Lithium borohydride (LiBH4) is a tetrahydroborate and known in organic synthesis as a reducing agent for esters. Although less common than the related sodium borohydride, the lithium salt offers some advantages, being a stronger reducing agent and highly soluble in ethers, whilst remaining safer to handle than lithium aluminium hydride.[2]
Preparation
Lithium borohydride may be prepared by the metathesis reaction, which occurs upon ball-milling the more commonly available sodium borohydride, and lithium bromide:[3]
- NaBH4 + LiBr → NaBr + LiBH4
Alternatively it may be synthesized by treating boron trifluoride with lithium hydride in diethyl ether:[4]
- BF3 + 4 LiH → LiBH4 + 3 LiF
Reactions
Lithium borohydride is a stronger reducing agent than sodium borohydride.[5] In mixtures of methanol and diethyl ether, lithium borohydride is able to reduce esters to alcohols and primary amides to amines.[6] In contrast, these substrates are unaffected by sodium borohydride. The enhanced reactivity is attributed to the polarization of the carbonyl substrate by complexation to the lithium cation.[2]
Chemoselectivity
The use of lithium borohydride is particularly advantageous in some preparations due to its higher chemoselectivity relative to other popular reducing agents such as lithium aluminium hydride. For instance, unlike lithium aluminium hydride, lithium borohydride will reduce esters, nitriles, lactones, primary amides, and epoxides while sparing nitro groups, carbamic acids, alkyl halides, and secondary/tertiary amides.[6]
Hydrogen Generation
Lithium borohydride reacts with water to produce hydrogen. This reaction can be used for hydrogen generation.[7]
Energy storage

Lithium borohydride is renowned as one of the highest energy density chemical energy carriers. Although presently of no practical importance, the solid will liberate 65 MJ/kg heat upon treatment with atmospheric oxygen. Since it has a density of 0.67 g/cm3, oxidation of liquid lithium borohydride gives 43 MJ/L. In comparison, gasoline gives 44 MJ/kg (or 35 MJ/L), while liquid hydrogen gives 120 MJ/kg (or 8.0 MJ/L).[nb 1] The high specific energy density of lithium borohydride has made it an attractive candidate to propose for automobile and rocket fuel, but despite the research and advocacy it has not been used widely. As with all chemical-hydride-based energy carriers, lithium borohydride is very complex to recycle (i.e. recharge) and therefore suffers from a low energy conversion efficiency. While batteries such as lithium ion carry an energy density of up to 0.72 MJ/kg and 2.0 MJ/L, their DC to DC conversion efficiency can be as high as 90% . In view of the complexity of recycling mechanisms for metal hydrides,[8] such high energy conversion efficiencies are beyond practical reach.
| Substance | Specific energy MJ/kg | Density g/cm3 | Energy density MJ/L |
|---|---|---|---|
| LiBH4 | 65.2 | 0.666 | 43.4 |
| Regular gasoline | 44 | 0.72 | 34.8 |
| Liquid hydrogen | 120 | 0.0708 | 8 |
| lithium ion battery | 0.72 | 2.8 | 2 |
See also
Notes
- ↑ The greater ratio of energy density to specific energy for hydrogen is because of the very low mass density (0.071 g/cm3).
References
- ↑ Sigma-Aldrich Product Detail Page
- 1 2 Luca Banfi, Enrica Narisano, Renata Riva, Ellen W. Baxter "Lithium Borohydride" e-EROS Encyclopedia of Reagents for Organic Synthesis, 2001 John Wiley & Sons. doi:10.1002/047084289X.rl061.pub2.
- ↑ Peter Rittmeyer, Ulrich Wietelmann “Hydrides” in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_199
- ↑ Brauer, Georg (1963). Handbook of Preparative Inorganic Chemistry Vol. 1, 2nd Ed. Newyork: Academic Press. p. 775. ISBN 978-0121266011.
- ↑ Barrett, Anthony G.M. (1991). "Reduction of Carboxylic Acid Derivatives to Alcohols, Ethers and Amines". In Trost, Barry; Fleming, Ian; Schreiber, Stuart. Reduction: Selectivity, Strategy & Efficiency in Modern Organic Chemistry (1st ed.). Newyork: Pergamon Press. p. 244. doi:10.1016/B978-0-08-052349-1.00226-2. ISBN 9780080405995.
- 1 2 Ookawa, Atsuhiro; Soai, Kenso (1986). "Mixed solvents containing methanol as useful reaction media for unique chemoselective reductions within lithium borohydride". The Journal of Organic Chemistry. 51 (21): 4000–4005. doi:10.1021/jo00371a017.
- ↑ Y. Kojima et al., "Hydrogen Generation by Hydrolysis Reaction of Lithium Borohydride," International Journal of Hydrogen Energy, 29(12):1213-1217, August 2004; DOI: 10.1016/j.ijhydene.2003.12.009 sciencedirect link
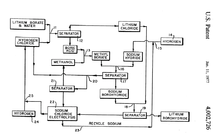
- ↑ US Patent 4002726 (1977) lithium borohydride recycling from lithium borate via a methyl borate intermediate