Marine bacteriophage
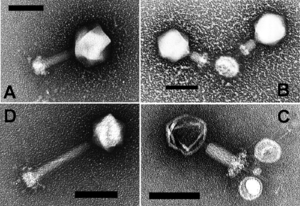
Marine bacteriophages or marine phages are viruses that live as obligate parasitic agents in marine bacteria such as cyanobacteria.[1] Their existence was discovered through electron microscopy and epifluorescence microscopy of ecological water samples, and later through metagenomic sampling of uncultured viral samples.[1][2] The tailed bacteriophages appear to dominate marine ecosystems in number and diversity of organisms.[1] However, viruses belonging to families Corticoviridae,[3] Inoviridae[4] and Microviridae[5] are also known to infect diverse marine bacteria. Metagenomic evidence suggests that microviruses (icosahedral ssDNA phages) are particularly prevalent in marine habitats.[5]
Bacteriophages, viruses that are parasitic on bacteria, were first discovered in the early twentieth century. Scientists today consider that their importance in ecosystems, particularly marine ecosystems, has been underestimated, leading to these infectious agents being poorly investigated and their numbers and species biodiversity being greatly under reported.[6]
Marine phages
Marine phages, although microscopic and essentially unnoticed by scientists until recently, appear to be the most abundant and diverse form of DNA replicating agent on the planet. There are approximately 4x1030 phage in oceans or 5x107 per millilitre.[7] HTVC010P infects one of the most common marine bacteria, Pelagibacter ubique of the SAR11 clade.[8][9] They appear to influence biogeochemical cycles globally, provide and regulate microbial biodiversity, cycle carbon through marine food webs, and are essential in preventing bacterial population explosions.[10] Scientists are exploring the potential of marine cyanophages to be used to prevent or reverse eutrophication.
In sediments
Marine bacteriophage form an important part of deep sea ecosystems. There are between 5x1012 and 1x1013 phage per square metre in deep sea sediments and their abundance closely correlates with the number of prokaryotes found in the sediments. They are responsible for the death of 80% of the prokaryotes found in the sediments, and almost all of these deaths are caused by cell lysis (bursting). They therefore play an important part in shifting nutrients from living forms into dissolved organic matter and detritus. This explains the high rate of nutrient turnover in deep sea sediments. The release of nutrients from infected bacteria stimulates the growth of uninfected bacteria and then these also become infected. Because of the importance of deep sea sediments in biogeochemical cycles, marine bacteriophage must influence the carbon, nitrogen and phosphorus cycles, but the exact influences are currently not understood.[7]
Carbon cycle
Marine viruses may play an important role in the carbon cycle by increasing the efficiency of the biological pump. The argument is that lysis releases unstable compounds, such as amino acids and nucleic acids, which tend to be recycled near the surface, whereas more indigestible carbon-rich material, such as that found in cell walls, is probably exported to deeper waters. Thus, the material that is exported to deeper waters by the 'viral shunt' is probably more carbon rich than the material from which it was derived. This would increase the efficiency of the biological pump.[11]
References
- 1 2 3 Mann, NH (2005-05-17). "The Third Age of Phage". PLoS Biol. United States: Public Library of Science. 3 (5): 753–755. doi:10.1371/journal.pbio.0030182. PMC 1110918
 . PMID 15884981.
. PMID 15884981. - ↑ Wommack, K. Eric; Russell T. Hill; Terri A. Muller; Rita R. Colwell (April 1996). "Effects of sunlight on bacteriophage viability and structure". Applied and Environmental Microbiology. United States of America: American Society for Microbiology. 62 (4): 1336–1341. PMC 167899
 . PMID 8919794.
. PMID 8919794. - ↑ Krupovic M, Bamford DH (2007). "Putative prophages related to lytic tailless marine dsDNA phage PM2 are widespread in the genomes of aquatic bacteria". BMC Genomics. 8: 236. doi:10.1186/1471-2164-8-236. PMC 1950889
 . PMID 17634101.
. PMID 17634101. - ↑ Xue H, Xu Y, Boucher Y, Polz MF (2012). "High frequency of a novel filamentous phage, VCY φ, within an environmental Vibrio cholerae population". Appl Environ Microbiol. 78 (1): 28–33. doi:10.1128/AEM.06297-11. PMC 3255608
 . PMID 22020507.
. PMID 22020507. - 1 2 Roux S, Krupovic M, Poulet A, Debroas D, Enault F (2012). "Evolution and diversity of the Microviridae viral family through a collection of 81 new complete genomes assembled from virome reads". PLoS ONE. 7 (7): e40418. doi:10.1371/journal.pone.0040418. PMC 3394797
 . PMID 22808158.
. PMID 22808158. - ↑ Kellogg, CA; JB Rose; SC Jiang; and JM Thurmond; JH Paul (1995). "Genetic diversity of related vibriophages isolated from marine environments around Florida and Hawaii, USA". Marine Ecology Progress Series. Germany: Inter-Research Science Center. 120 (1–3): 89–98. doi:10.3354/meps120089.
- 1 2 Danovaro, Roberto; Dell'Anno, Antonio; Corinaldesi, Cinzia; Magagnini, Mirko; Noble, Rachel; Tamburini, Christian; Weinbauer, Markus (2008-08-28). "Major viral impact on the functioning of benthic deep-sea ecosystems". Nature. 454 (7208): 1084–1087. doi:10.1038/nature07268. PMID 18756250. Retrieved 2009-05-03.
- ↑ Zhao, Y.; Temperton, B.; Thrash, J. C.; Schwalbach, M. S.; Vergin, K. L.; Landry, Z. C.; Ellisman, M.; Deerinck, T.; Sullivan, M. B.; Giovannoni, S. J. (2013). "Abundant SAR11 viruses in the ocean". Nature. 494 (7437): 357–360. doi:10.1038/nature11921. PMID 23407494.
- ↑ Kirchman, D. L. (2013). "Microbial oceanography: Killers of the winners". Nature. 494 (7437): 320–1. doi:10.1038/nature11951. PMID 23407493.
- ↑ Waldor, M. K.; Friedman, D. I.; Adhya, S. L., eds. (2005). Phages: their role in bacterial pathogenesis and biotechnology. Washington DC: ASM Press. p. 450. ISBN 978-1-55581-307-9.
- ↑ Suttle CA. Marine viruses—major players in the global ecosystem. Nature Reviews Microbiology. October 2007;5(10):801–12. doi:10.1038/nrmicro1750. PMID 17853907.


