Noonan syndrome
| Noonan syndrome | |
|---|---|
|
| |
| A 12-year-old girl with Noonan syndrome. Typical webbed neck. Double structural curve with rib deformity. | |
| Classification and external resources | |
| Specialty | Medical genetics, pediatrics |
| ICD-10 | Q87.1 |
| ICD-9-CM | 759.89 |
| OMIM | 163950 605275 609942 610733 611553 |
| DiseasesDB | 29094 |
| MedlinePlus | 001656 |
| eMedicine | article/947504 |
| Patient UK | Noonan syndrome |
| MeSH | D009634 |
| GeneReviews | |
Noonan syndrome (NS) is a relatively common autosomal dominant congenital disorder and is named after Jacqueline Noonan, a pediatric cardiologist. It is referred to as the male version of Turner's syndrome,[1][2] however the genetic causes of Noonan syndrome and Turner syndrome are distinct. The principal features include congenital heart defect (typically pulmonary valve stenosis; also atrial septal defect and hypertrophic cardiomyopathy), short stature, learning problems, pectus excavatum, impaired blood clotting, and a characteristic configuration of facial features including a webbed neck and a flat nose bridge. NS is a RASopathy, and is one of several disorders that are caused by a disruption of RAS-MAPK signaling pathway.
It is believed that between approximately 1 in 1,000 and 1 in 2,500 children worldwide are born with NS. It is one of the most common genetic syndromes associated with congenital heart disease, similar in frequency to Down syndrome. However, the range and severity of features can vary greatly in patients with NS. Therefore, the syndrome is not always identified at an early age.
Characteristics
Organ system
Anesthesia risk
- There have been few reports of patients with Noonan syndrome who have subsequently suffered from Malignant Hyperthermia (MH). A similar genetic disease, King-Denborough syndrome, has a high risk for MH. King-Denborough syndrome has been linked to a mutation on chromosome 19 located near the gene that encodes the ryanodine receptor whereas Noonan syndrome is associated with a mutation on chromosome 12.[3]
- Some individuals with NS have been reported to develop respiratory depression from narcotics given during and after surgery, which has sometimes required cardiac resuscitation. Impaired anesthetic clearance in the 24–48 hours after surgery has also been reported.
Heart
Up to ~85% of people with NS have one of the following heart defects:
- Pulmonary valvular stenosis (50–60%)
- Septal defects: atrial (10–25%) or ventricular (5–20%)
- Hypertrophic cardiomyopathy (12–35%)
Lungs
- Restrictive lung function has been reported in some patients
Gastrointestinal system
- Failure to thrive From infancy to puberty (75%)
- Decreased appetite
- Digestive problems
- Frequent or forceful vomiting
- Swallowing difficulties
- Intestinal malrotation
- Need for a feeding tube
- Low gut motility
- Gastroparesis (delayed gastric emptying)
Genito-urinary system
- Cryptorchidism (undescended testicles)
Lymphatic system
- Posterior cervical hygroma (webbed neck)
- Lymphedema
Developmental
- Clumsiness
- Motor development delay/delayed milestones
- Learning disabilities
- Dysmaturity
- Speech-language pathology
- Autism, pervasive developmental disorder
- Frequent illnesses, doctor appointments, pain, headaches and fatigue are a few things that can affect school attendance and performance.
- Despite the various possible developmental/learning issues that can be seen in NS, there are Noonan Syndrome patients that have advanced degrees, including at least two that have become attorneys. Source Noonan Syndrome Foundation
Recommendations
- Neuropsychological testing is recommended to find strengths and challenges to tailor support needed for school, career.
- Educational customization such as an Individualized Education Program plan or a Section 504 plan is sometimes needed for school-aged children.
- Speech therapy if there are speech and articulation issues
- Physical therapy and occupational therapy for gross and fine motor delays
- Hypotonia and motor difficulties often impact handwriting. Accommodations for lessening handwriting demands will improve performance and save long-term hand function.
Hematologic
- Bleeding disorders
- Easy bruising
- Amegakaryocytic thrombocytopenia (low platelet count)
- Blood clotting disorders
- Von Willebrand disease
- Prolonged activated partial thromboplastin time
- Partial deficiency of Factor VIII:C
- Partial deficiency of Factor XI:C
- Partial deficiency of Factor XII:C
- Platelet dysfunction
- Combined coagulation defects
- Imbalance of Plasminogen Activator Inhibitor Type-1 (PAI-1) and Tissue Plasminogen Activator (t-PA) Activity.[4]
Musculoskeletal
- Joint pain or muscle pain especially in adults, which can vary in severity[5]
- Undifferentiated Connective Tissue Disorders
- Scoliosis
- Prominence of breast bone (pectus carinatum)
- Depression of breast bone (pectus excavatum)
- Joint contractures (tightness)
- Joint hypermobility (looseness)
- Winging of the scapula
- Hypotonia (low muscle tone)
- Hypermobility syndrome
- Lordosis (increased hollow in the back) due to poor stomach muscle tone
Neurological
- Arnold-Chiari malformation (type 1), which can lead to hydrocephalus, has been noted in some patients
- Seizures
Physical appearance
Stature
- Short stature Growth Hormone (GH) sometimes combined with IGF-1 (or as an alternative, IGF-1 as a stand-alone could be used as stated in cited paper) can be used to achieve an increased height/final height quicker [6][7][8]
Head
- Excess skin on the back of the neck
- Low hairline at the nape of the neck
- High hairline at the front of the head
- Large head
- Triangular face shape
- Broad forehead
- Short neck, webbed neck
Eyes
- Hypertelorism (widely set eyes) (95%)
- Epicanthal folds (extra fold of skin at the inner corner of the eye)
- Ptosis (drooping of the eyelids)
- Proptosis (bulging eyes)
- Refractive visual errors
- Strabismus (inward or outward turning of the eyes)
- Nystagmus (jerking movement of the eyes)
Nose
- Small, upturned nose
Ears and hearing
- Low-set ears (in over 90%)
- Backward-rotated ears (over 90%)
- Thick helix (outer rim) of ear(over 90%)
- Incomplete folding of ears
- Chronic otitis media (ear infections)
- Hearing loss
Mouth and speech
- Deeply grooved philtrum (top lip line) (over 90%)
- Micrognathia (undersized lower jaw)
- High arched palate
- Dental problems
- Articulation difficulties
- Poor tongue control
Limbs/extremities
- Bluntly ended fingers
- Extra padding on fingers and toes
- Edema of the back of hands and tops of feet
- Cubitus valgus (Wide carrying angle of the elbows)
Skin
- Lymphedema (lymph swelling of the extremities)
- Keloid formation, excessive scar formation
- Hyperkeratosis (overdevelopment of outer skin layer)
- Pigmented nevi (darkly pigmented skin spots)
- Connective tissue disease
Causes
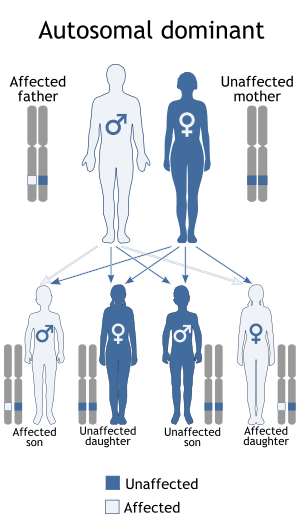
Recurrence in siblings and apparent transmission from parent to child has long suggested a genetic defect with autosomal dominant inheritance and variable expression. Mutations in the Ras/mitogen activated protein kinase signaling pathways are known to be responsible for ~70% of NS cases.[9]
A person with NS has up to a 50% chance of transmitting it to their offspring. The fact that an affected parent is not always identified for children with NS suggests several possibilities:
- Manifestations could be so subtle as to go unrecognized (variable expressivity)
- NS is heterogeneous, comprising more than one similar condition of differing causes, and some of these may not be inherited.
- A high proportion of cases may represent new, sporadic mutations.
| Type | Online Mendelian Inheritance in Man database | Gene | Year found | Locus | % of cases | Description | Refs. |
|---|---|---|---|---|---|---|---|
| NS1 | 163950 | PTPN11 | 2001 | 12q24.1 | 50% | The PTPN11 gene encodes the protein tyrosine phosphatase SHP-2. This protein is a component of several intracellular signal transduction pathways involved in embryonic development that modulate cell division, differentiation, and migration, including one mediated by the epidermal growth factor receptor, which is important in the formation of the semilunar heart valves. Duplication of the chromosome region containing PTPN11 can also result in NS. |
[10] [11] |
| NS2 | 605275 | Unknown; autosomal recessive | [12] | ||||
| NS3 | 609942 | KRAS | 2006 | 12p12.1 | <5% | [13] | |
| NS4 | 610733 | SOS1 | 2006 | 2p21 | 10% | Activating mutations in SOS1 can give rise to NS. SHP-2 and SOS1 positively regulate the Ras/MAP kinase pathway, suggesting that its dysregulation mediates NS development.[14] | [15] |
| NS5 | 611553 | RAF1 | 2007 | 3p25 | 3–17% | [16] |
Heterozygous mutations in NRAS, HRAS, BRAF, SHOC2, MAP2K1, MAP2K2, and CBL have also been associated with a smaller percentage of NS and related phenotypes.[17]
A condition known as "neurofibromatosis-Noonan syndrome" is associated with neurofibromin.[18]
Diagnosis
NS can be confirmed genetically by the presence of any of the known mutations listed above. However, despite identification of fourteen causative genes, the absence of a known mutation will not exclude the diagnosis, as there are more, as-yet-undiscovered genes that cause NS. Thus, the diagnosis of NS is still based on clinical features. In other words, it is made when a physician feels that a patient has enough of the features to warrant the label. The principal values of making a genetic diagnosis are that it guides additional medical and developmental evaluations, it excludes other possible explanations for the features, and it allows more accurate recurrence risk estimates. With more genotype-phenotype correlation studies being performed, a positive genetic diagnosis will help the clinician to be aware of possible anomalies specific to that certain gene mutation. For example, there is an increase in hypertrophic cardiomyopathy in patients with a mutation of KRAS and an increased risk of juvenile myelomonocytic leukemia for a mutation of PTPN11. In the future, studies may lead to a targeted management of NS symptoms that depends on what genetic mutation a patient has.
Prognosis
A 2007 study followed 112 individuals for a mean of 12 years (mean age 25.3, range 12-71). No patient died during follow-up, but several required medical interventions. The mean final heights were 167 and 153 cm for men and women, respectively, which is approximately 2 standard deviations below normal.[19]
History
Jacqueline Noonan was practicing as a pediatric cardiologist at the University of Iowa when she noticed that children with a rare type of heart defect, valvular pulmonary stenosis, often had a characteristic physical appearance, with short stature, webbed neck, wide spaced eyes, and low-set ears. Both boys and girls were affected. These characteristics were sometimes seen running in families but were not associated with gross chromosomal abnormalities. She studied 833 patients at the congenital heart disease clinic, looking for other congenital abnormalities, and in 1963 presented a paper: "Associated non-cardiac malformations in children with congenital heart disease". This described 9 children who in addition to congenital heart disease had characteristic facial features, chest deformities and short stature.
Dr. John Opitz, a former student of Dr. Noonan, first began to call the condition "Noonan syndrome" when he saw children who looked like those whom Dr. Noonan had described. Dr. Noonan produced a paper entitled "Hypertelorism with Turner Phenotype" in 1968,[20] and in 1971 at the Symposium of Cardiovascular defects, the name 'Noonan syndrome' became officially recognized.
See also
- Turner syndrome, a different disorder often confused with NS because of several physical features that they share
- Fetal alcohol syndrome, another disorder sometimes confused with NS because of some common facial features and mental retardation[21]
- Other RASopathies, particularly:
- Costello syndrome
- Legius syndrome
- Noonan Syndrome with Multiple Lentigines, as known as LEOPARD syndrome, a related disorder caused by mutations in PTPN11 that are catalytically inactivating.
- Cardiofaciocutaneous syndrome, a related disorder which also affects genes encoding elements of the Ras/MAP kinase pathway.
- Dermatoglyphics
References
- ↑ Curcić-Stojković O, Nikolić L, Obradović D, Krstić A, Radić A (1978). "[Noonan's syndrome. (Male Turner's syndrome, Turner-like syndrome)]". Med Pregl. 31 (7–8): 299–303. PMID 692497.
- ↑ "Noonan syndrome" at Dorland's Medical Dictionary
- ↑ "Does Noonan Syndrome Increase Malignant Hyperthermia Susceptibility?". Malignant Hyperthermia Association of the United States. Retrieved 24 October 2014.
- ↑ "Imbalance of plasminogen activator inhibitor type-1 (PAI-1) and tissue plasminogen activator (t-PA) activity in patients with Noonan syndrome.". J Pediatr Hematol Oncol. 32 (7): 532–6. Oct 2010. doi:10.1097/MPH.0b013e3181e0d152. PMID 20686427.
- ↑ Reinker, Kent; Stevenson DA; Tsung A (July–August 2011). "Orthopaedic conditions in Ras/MAPK related disorders.". Journal of Pediatric Orthopeadics. 31 (5): 599–605. doi:10.1097/BPO.0b013e318220396e. PMID 21654472.
- ↑ Growth hormone and noonan syndrome: update in dysfunctional signaling aspects and in therapy for short stature
- ↑ "Growth hormone and noonan syndrome: update in dysfunctional signaling aspects and in therapy for short stature". Hormonal Studies. 2: 1. doi:10.7243/2052-8000-2-1.
- ↑ http://www.hoajonline.com/hormones/2052-8000/2/1
- ↑ Razzaque MA, Komoike Y, Nishizawa T, et al. (March 2012). "Characterization of a novel KRAS mutation identified in Noonan syndrome". Am. J. Med. Genet. A. 158A (3): 524–32. doi:10.1002/ajmg.a.34419. PMID 22302539.
- ↑ Tartaglia M, Mehler EL, Goldberg R, et al. (2001). "Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome". Nat. Genet. 29 (4): 465–8. doi:10.1038/ng772. PMID 11704759.
- ↑ Shchelochkov OA, Patel A, Weissenberger GM, et al. (April 2008). "Duplication of chromosome band 12q24.11q24.23 results in apparent Noonan syndrome". Am. J. Med. Genet. A. 146A (8): 1042–8. doi:10.1002/ajmg.a.32215. PMID 18348260.
- ↑ Van Der Burgt, I.; Brunner, H. (2000). "Genetic heterogeneity in Noonan syndrome: Evidence for an autosomal recessive form". American Journal of Medical Genetics. 94 (1): 46–51. doi:10.1002/1096-8628(20000904)94:1<46::AID-AJMG10>3.0.CO;2-I. PMID 10982482.
- ↑ Schubbert S, Zenker M, Rowe SL, et al. (2006). "Germline KRAS mutations cause Noonan syndrome". Nat. Genet. 38 (3): 331–6. doi:10.1038/ng1748. PMID 16474405.
- ↑ Bentires-Alj M, Kontaridis MI, Neel BG (2006). "Stops along the RAS pathway in human genetic disease". Nat. Med. 12 (3): 283–5. doi:10.1038/nm0306-283. PMID 16520774.
- ↑ Roberts AE, Araki T, Swanson KD, et al. (2007). "Germline gain-of-function mutations in SOS1 cause Noonan syndrome". Nat. Genet. 39 (1): 70–4. doi:10.1038/ng1926. PMID 17143285.
- ↑ Razzaque MA, Nishizawa T, Komoike Y, et al. (2007). "Germline gain-of-function mutations in RAF1 cause Noonan syndrome". Nat. Genet. 39 (8): 1013–7. doi:10.1038/ng2078. PMID 17603482.
- ↑ http://www.mayomedicallaboratories.com/interpretive-guide/?alpha=N&unit_code=61851
- ↑ De Luca, A.; Bottillo, I.; Sarkozy, A.; Carta, C.; Neri, C.; Bellacchio, E.; Schirinzi, A.; Conti, E.; Zampino, G.; Battaglia, A.; Majore, S.; Rinaldi, M. M.; Carella, M.; Marino, B.; Pizzuti, A.; Digilio, M. C.; Tartaglia, M.; Dallapiccola, B. (2005). "NF1 gene mutations represent the major molecular event underlying neurofibromatosis-Noonan syndrome". American Journal of Human Genetics. 77 (6): 1092–1101. doi:10.1086/498454. PMC 1285166
 . PMID 16380919.
. PMID 16380919. - ↑ Shaw, A. C.; Kalidas, K.; Crosby, A. H.; Jeffery, S.; Patton, M. A. (2007-02-01). "The natural history of Noonan syndrome: a long-term follow-up study". Archives of Disease in Childhood. 92 (2): 128–132. doi:10.1136/adc.2006.104547. ISSN 1468-2044. PMC 2083343
 . PMID 16990350.
. PMID 16990350. - ↑ Noonan, JA (1968). "Hypertelorism with Turner phenotype. A new syndrome with associated congenital heart disease". Am. J. Dis. Child. 116 (4): 373–80. doi:10.1001/archpedi.1968.02100020377005. PMID 4386970.
- ↑ CDC. (2004). Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis. Can be downloaded at http://www.cdc.gov/fas/faspub.htm.
External links
| Wikimedia Commons has media related to Noonan syndrome. |