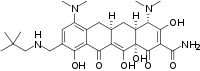
Omadacycline
 | |
| Identifiers | |
|---|---|
| 389139-89-3 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 20131003 |
| PubChem | 54697325 |
| UNII | 090IP5RV8F |
| |
| |
| Properties | |
| C29H40N4O7 | |
| Molar mass | 556.66 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Omadacycline (formerly known as PTK-0796)[1] is a broad spectrum antibiotic belonging to the aminomethylcycline subclass[2] of tetracycline antibiotics. Paratek Pharmaceuticals is developing omadacycline as a treatment for serious community-acquired infections.
In vitro Studies
In vitro studies have shown that omadacycline has activity against a broad range of Gram-positive and select Gram-negative pathogens.[3] Omadacycline has potent In Vitro activity against Gram-positive aerobic bacteria including methicillin-resistant Staphylococcus aureus (MRSA), pencillin resistant and multi-drug resistant Streptococcus pneumoniae and vancomycin-resistant Enterococcus. Omadacycline has also shown activity against common Gram-negative aerobes, some anaerobes and atypical bacteria such as Legionella and Chlamydia.[4] This activity translated to potent efficacy for omadacycline in an In vivo systemic infection model in mice.[5]
Additional in vitro and in vivo studies of omadacycline metabolism, dispositon and drug-drug interactions show that omadacycline is metabolically stable (no significant biotransformations) and does not inhibit or interact with metabolizing enzymes or transporters.[6]
Mechanism of Action
The mechanism of action for omadacycline has been shown to be primarily through inhibition of bacterial protein synthesis. Omadacycline has demonstrated activity against bacterial strains expressing the two main forms of tetracycline resistance (efflux and ribosomal protection).[7]
Clinical Trials
A Phase 2 study was conducted comparing the safety and efficacy of omadacycline to linezolid for the treatment of complicated skin and skin structure infections (cSSSI). Patients were randomized to receive either omadacycline 100 mg intravenously once daily with an option to transition to 200 mg orally once daily or linezolid 600 mg intravenously twice daily with an option to transition to 600 mg orally twice daily at 11 US sites. The phase 2 trial results support conclusions that omadacycline is well tolerated in cSSSI patients and has the potential to be an effective treatment for serious skin infections.[8]
In June 2013, the US Food and Drug Administration (FDA) designated omadacycline as a Qualified Infectious Disease Product (QIDP) for both intravenous and oral formulations in the treatment of acute bacterial skin and skin structure infections (ABSSSI) and community-acquired bacterial pneumonia (CABP).[9]
A 650 patient Phase 3 registration study known as OASIS (Omadacycline in Acute Skin and Skin Structure Infections Study), comparing omadacycline to linezolid for the treatment of Acute Bacterial Skin and Skin Structure Infections (ABSSSI) began in June 2015.[10][11] Omadacycline met the primary efficacy endpoint of early clinical response with statistical non-inferiority (10% margin) compared to linezolid. Omadacycline was generally safe and well tolerated. The most common treatment emergent adverse event was gastrointestinal events (18.0% for omadacycline vs. 15.8% for linezolid).[12]
A 750 patient Phase 3 study comparing omadacycline to moxifloxacin for the treatment of Community Acquired Bacterial Pneumonia (CABP) began in November 2015.[13]
In May 2016, a phase 1b study of omadacycline in Urinary Tract Infection was initiated.[14]
In August 2016, a second, pivotal phase 3 study of omadacycline in patients with ABSSSI was initiated. The study will assess the efficacy and safety of once-daily, oral-only omadacycline compared to twice-daily, oral-only linezolid in patients with ABSSSI.[15]
References
- ↑ http://www.bioworld.com/content/antibiotic-firm-paratek-joins-ipo-queue-aiming-92m-0
- ↑ Honeyman, Laura; Ismail, Mohamed; Nelson, Mark L.; Bhatia, Beena; Bowser, Todd E.; Chen, Jackson; Mechiche, Rachid; Ohemeng, Kwasi; Verma, Atul K.; Cannon, E. Pat; MacOne, Ann; Tanaka, S. Ken; Levy, Stuart (2015). "Structure-Activity Relationship of the Aminomethylcyclines and the Discovery of Omadacycline". Antimicrobial Agents and Chemotherapy. 59 (11): 7044. doi:10.1128/AAC.01536-15. PMID 26349824.
- ↑ Tanaka, S. Ken (20 June 2016). "In Vitro and In Vivo Assessment of Cardiovascular Effects with Omadacycline". Antimicrob. Agents Chemother. 60 (9): 5247–53. doi:10.1128/AAC.00320-16. PMID 27324778.
- ↑ Villano, Stephen (19 August 2016). "Omadacycline: development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections". Future Microbiology. doi:10.2217/fmb-2016-0100. Retrieved 24 August 2016.
- ↑ MacOne, A. B.; Caruso, B. K.; Leahy, R. G.; Donatelli, J.; Weir, S.; Draper, M. P.; Tanaka, S. K.; Levy, S. B. (2013). "In Vitro and in Vivo Antibacterial Activities of Omadacycline, a Novel Aminomethylcycline". Antimicrobial Agents and Chemotherapy. 58 (2): 1127. doi:10.1128/AAC.01242-13. PMID 24295985.
- ↑ Flarakos, Jimmy (8 Aug 2016). "Clinical disposition, metabolism and in vitro drug–drug interaction properties of omadacycline". Xenobiotica: 1. doi:10.1080/00498254.2016.1213465.
- ↑ Draper, M. P.; Weir, S.; MacOne, A.; Donatelli, J.; Trieber, C. A.; Tanaka, S. K.; Levy, S. B. (2013). "Mechanism of Action of the Novel Aminomethylcycline Antibiotic Omadacycline". Antimicrobial Agents and Chemotherapy. 58 (3): 1279. doi:10.1128/AAC.01066-13. PMID 24041885.
- ↑ Noel, G. J.; Draper, M. P.; Hait, H.; Tanaka, S. K.; Arbeit, R. D. (2012). "A Randomized, Evaluator-Blind, Phase 2 Study Comparing the Safety and Efficacy of Omadacycline to Those of Linezolid for Treatment of Complicated Skin and Skin Structure Infections". Antimicrobial Agents and Chemotherapy. 56 (11): 5650. doi:10.1128/AAC.00948-12. PMID 22908151.
- ↑ http://www.prnewswire.com/news-releases/paratek-pharmaceuticals-announces-fda-grant-of-qualified-infectious-disease-product-qidp-designation-for-its-lead-product-candidate-omadacycline-185554432.html
- ↑ http://www.bizjournals.com/boston/blog/bioflash/2016/04/paratek-presents-new-trial-data-for-antibiotic-as.html
- ↑ "Omadacycline Versus Linezolid for the Treatment of ABSSSI (EudraCT #2013-003644-23) - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2015-10-13.
- ↑ "Paratek Announces that Omadacycline Met All Primary and Secondary Efficacy Outcomes Designated by FDA and EMA in a Phase 3 Study in Acute Bacterial Skin Infections; Omadacycline was Generally Safe and Well-Tolerated". finance.yahoo.com. Retrieved 3 July 2016.
- ↑ "Omadacycline vs Moxifloxacin for the Treatment of CABP (EudraCT #2013-004071-13) - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2015-10-13.
- ↑ "Paratek Initiates Phase 1b Study of Omadacycline in Urinary Tract Infection". globenewswire.com. Retrieved 3 July 2016.
- ↑ "Paratek Initiates Phase 3 Study of Oral-only Omadacycline in ABSSSI". globenewswire.com. Retrieved 15 August 2016.
