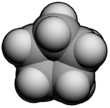
Cyclopentane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Cyclopentane | |||
| Other names
pentamethylene | |||
| Identifiers | |||
| 287-92-3 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:23492 | ||
| ChemSpider | 8896 | ||
| ECHA InfoCard | 100.005.470 | ||
| EC Number | 206-016-6 | ||
| PubChem | 9253 | ||
| RTECS number | GY2390000 | ||
| |||
| |||
| Properties | |||
| C5H10 | |||
| Molar mass | 70.1 g/mol | ||
| Appearance | clear, colorless liquid | ||
| Odor | mild, sweet | ||
| Density | 0.751 g/cm3 | ||
| Melting point | −93.9 °C (−137.0 °F; 179.2 K) | ||
| Boiling point | 49.2 °C (120.6 °F; 322.3 K) | ||
| 156 mg·l−1 (25 °C)[1] | |||
| Solubility | soluble in ethanol, acetone, ether | ||
| Vapor pressure | 400 mmHg (31 °C)[2] | ||
| Acidity (pKa) | ~45 | ||
| Refractive index (nD) |
1.4065 | ||
| Hazards | |||
| Main hazards | Flammable[2] | ||
| NFPA 704 | |||
| Flash point | −37.2 °C (−35.0 °F; 236.0 K) | ||
| 361 °C (682 °F; 634 K) | |||
| Explosive limits | 1.1%-8.7%[2] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
none[2] | ||
| REL (Recommended) |
TWA 600 ppm (1720 mg/m3)[2] | ||
| IDLH (Immediate danger) |
N.D.[2] | ||
| Related compounds | |||
| Related compounds |
cyclopropane, cyclobutane, cyclohexane | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Cyclopentane is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occurs as a colorless liquid with a petrol-like odor. Its melting point is −94 °C and its boiling point is 49 °C. Cyclopentane is in the class of cycloalkanes, being alkanes that have one or more rings of carbon atoms.
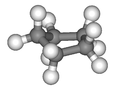
It is formed by cracking cyclohexane in the presence of alumina at a high temperature and pressure.
It was first prepared in 1893 by the German chemist Johannes Wislicenus.[3]
Industrial usage
Cyclopentane is used in the manufacture of synthetic resins and rubber adhesives and also as a blowing agent in the manufacture of polyurethane insulating foam, as found in many domestic appliances such as refrigerators and freezers, replacing environmentally damaging alternatives such as CFC-11 and HCFC-141b[4]
Multiply alkylated cyclopentane (MAC) lubricants have low volatility and are used in some specialty applications.[5]
The United States produces more than half a million kilograms of this chemical per year.
Formulation of cycloalkanes
Cycloalkanes can be formulated via a process known as catalytic reforming.
For example, 2-methylbutane can be reformed into cyclopentane, by use of a platinum catalyst. This is particularly well known in automobiles, as branched alkanes will burn much more readily.
References
- ↑ Record of cyclopentane in the GESTIS Substance Database of the IFA, accessed on 28 February 2015
- 1 2 3 4 5 6 "NIOSH Pocket Guide to Chemical Hazards #0171". National Institute for Occupational Safety and Health (NIOSH).
- ↑ J. Wislicenus and W. Hentschel (1893) "Der Pentamethenylalkohol und seine Derivate" (Cyclopentanol and its derivatives), Annalen der Chemie, 275 : 322-330; see especially pages 327-330. Wislicenus prepared cyclopentane from cyclopentanone ("Ketopentamethen"), which is prepared by heating calcium adipate.
- ↑ Greenpeace - Appliance Insulation
- ↑ Pennzane - lubrication technology