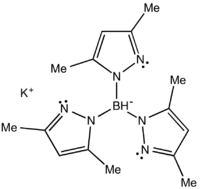
Potassium tris(3,5-dimethyl-1-pyrazolyl)borate
 | |
| Names | |
|---|---|
| IUPAC name
Potassium tri(3,5-dimethyl-1-pyrazolyl)borohydride | |
| Other names
Tp* ligand | |
| Identifiers | |
| 17567-17-8 | |
| ECHA InfoCard | 100.203.488 |
| Properties | |
| C15H22BKN6 | |
| Molar mass | 336.28 gmol-1 |
| Appearance | White solid |
| Melting point | 292 to 301 °C (558 to 574 °F; 565 to 574 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium tris(3,5-dimethyl-1-pyrazolyl)borate, abbreviated KTp*, is the potassium salt of the anion HB((CH3)2C3N2H)3. Tp*− is a tripodal ligand that binds to a metal in a facial manner, more specifically a Scorpionate ligand.[1] KTp* is a white crystalline solid that is soluble in polar solvents, including water and several alcohols.
Synthesis
KTp* is synthesized in a manner similar to that of KTp by the reaction of potassium borohydride and 3,5-dimethylpyrazole. Hydrogen gas is evolved as each of the pyrazole reacts at the boron. The rate of B-N bond formation becomes more difficult with each successive 3,5-dimethylpyrazolyl due to the increase in steric hindrance around the boron:[2]
- 3 Me2C3N2H2 + KBH4 → KHB(Me2C3N2H)3 + 3 H2
The required dimethylpyrazole is obtained by condensation of hydrazine and acetylacetone.
Role as ligand
The active binding sites in Tp*− are the three nitrogen centers that are not bonded to the boron. Although more weakly binding than cyclopentadienyl ligands, Tp*− is still a tightly coordinating. The benefit of Tp*− over its sister compound Tp− is the addition of the methyl groups on the pyrazolyl rings, which increases the steric hindrance of the ligand enough that only one Tp*− can bind to a metal. This leaves the remaining coordination sites available for catalysis.[3]
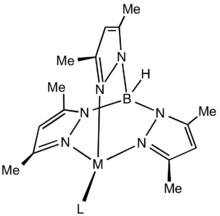 Structure of generic Tp*M complex illustrating the steric protection afforded by the methyl groups.
Structure of generic Tp*M complex illustrating the steric protection afforded by the methyl groups.
References
- ↑ Trofimenko, Swiatoslaw (1999). "Scorpionates: Polypyrazolylborate Ligands and Their Coordination Chemistry.". World Scientific Publishing Company.
- ↑ Trofimenko, S. (1970). "Poly(1-Pyrazolyl)Borates, Their Transition-Metal Complexes, and Pyrazaboles". Inorganic Syntheses. 12: 99–109. doi:10.1002/9780470132432.ch18.
- ↑ Trofimenko, S (2004). "Scorpionates: genesis, milestones, prognosis". Polyhedron. 23: 197–203. doi:10.1016/j.poly.2003.11.013.