Borohydride
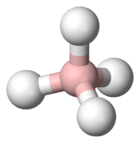
Borohydride refers to the anion BH4− and its salts.[1] Borohydride is also the term used for compounds containing BH4-nXn−, for example cyanoborohydride (B(CN)H3−) and triethylborohydride (B(C2H5)3H−). Such compounds find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known (see Table).[2] Tetrahydroborates are also of academic and industrial interest in inorganic chemistry.[3]
History
Alkali metal borohydrides were described in 1940 by Hermann Irving Schlesinger and Herbert C. Brown. They synthesized lithium borohydride (LiBH4) from diborane (B2H6):[4][5]
- 2 MH + B2H6 → 2 M[BH4] (M = Li, Na, K, etc.)
Current methods involve reduction of trimethyl borate with sodium hydride.[2]
Structure
The borohydride anion has a tetrahedral structure with boron in the center and the hydrogens located at the four vertices of the tetrahedron.[6] In metal complexes, the borohydride ion is bound to the metal by means of bridging hydrogen atoms, usually in one of three different ways: monodentate (one hydrogen bridge), bidentate (two hydrogen bridges), and tridentate (three hydrogen bridges)[7] (η1, η2, or η3).[3] The preferred coordination mode is strongly affected by the nature of the metal and its oxidation state.
| Hydride CAS registry number | Mol.Wt. | Hydrogen Density | Density g/cm3 | m.p. (°C) | solubility in water (g/100 mL, 25 °C) | solubility in CH3OH (g/100 mL, 25 °C) | solubility in ether (g/100 mL, 25 °C) | solubility in THF (g/100 mL, 25 °C) |
|---|---|---|---|---|---|---|---|---|
| LiBH4
[16949-15-8] |
21.78 | 18.5 | 0.66 | 280 | 20.9 | decomp. (44 in EtOH) | 4.3 | 22.5 |
| NaBH4
[16940-66-2] |
37.83 | 10.6 | 1.07 | 505 | 55 | 16.4 (20 °C) | insol. | 0.1 (20 °C) |
| NaBH3CN
[25895-60-7] |
62.84 | 6.4 | 1.20 | 240 with deccomp. | deccomp. | 217 | insol. | 36 |
| KBH4
[13762-51-1] |
53.94 | 7.4 | 1.17 | 585 (under H2) | 19 | insol. | insol. | insol. |
| LiBH(C2H5)3
[22560-16-3] |
105.94 | 0.95 | unknown | unknown | decomp. | decomp. | na | high (supplied commercially) |
Uses
Sodium borohydride is the borohydride that is produced on the largest scale industrially, estimated at 5000 tons/y in 2002. The main use is for the reduction of sulfur dioxide to give sodium dithionite:
- NaBH4 + 8 NaOH + 8 SO2 → 4 Na2S2O4 + NaBO2 + 6 H2O
Dithionite is used to bleach wood pulp.[2] Sodium borohydride is also used to reduce aldehydes and ketones in the production of pharmaceuticals including chloramphenicol, thiophenicol, vitamin A, atropine, and scopolamine, as well as many flavorings and aromas.
Potential applications
Because of their high hydrogen content, borohydride complexes and salts have been of interest in the context of hydrogen storage.[8] Reminiscent of related work on ammonia borane, challenges are associated with slow kinetics and low yields of hydrogen as well as problems with regeneration of the parent borohydrides.
Coordination complexes
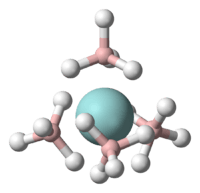
Borohydrides can serve as ligands for many metals, and there is a large coordination chemistry of the borohydride ion.[9] In most such compounds, the BH4− ligand is bidentate. Some binary borohydrides (i.e., containing only BH4− ligands) are highly volatile. One example is uranium borohydride.
Metal borohydride complexes can often be prepared by a simple salt elimination reaction:
- TiCl4 + LiBH4 → Ti(BH4)3(Et2O) in diethyl ether[10]
Decomposition
Some metal tetrahydroborates transform on heating to give metal borides. When the borohydride complex is volatile, this decomposition pathway is the basis of chemical vapor deposition, a way of depositing thin films of metal borides.[11] For example, zirconium and hafnium diborides, ZrB2 and HfB2, can be prepared through CVD of the tetrahydroborates Zr(BH4)4 and Hf(BH4)4:
M(BH4)4 → MB2 + B2H6 + 5H2 [11]
Metal diborides find uses as coatings because of their hardness, high melting point, strength, resistance to wear and corrosion, and good electrical conductivity.[11] The diboride films can be deposited on a variety of substances including glass, copper, aluminum, and steel.[11]
References
- ↑ "Tetrahydroborate". Retrieved 26 February 2013.
- 1 2 3 Rittmeyer, P.; Wietelmann, U. “Hydrides” in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_199
- 1 2 Makheav, V.D. Russ. Chem. Rev. 2000, 69, 727-746.doi:10.1070/RC2000v069n09ABEH000580
- ↑ Schlesinger, H.C.; Brown, H.R. "Metallo Borohydrides. III. Lithium Borohydride" J. Am. Chem. Soc. 1940, 62, 3429-3435. doi:10.1021/ja01869a039
- ↑ Schlesinger, H.C.; Brown, H.R.; Hoekstra, L.R. "Reactions of Diborane with Alkali Metal Hydrides and Their Addition Compounds. New Syntheses of Borohydrides. Sodium and Potassium Borohydrides" J. Am. Chem. Soc. 1953, 75, 199–204. doi:10.1021/ja01097a053
- ↑ Zuttel, A.; Borgschulte, A.; Orimo, S. Scripta Materialia 2007, 56, 823–828. doi:10.1016/j.scriptamat.2007.01.010
- ↑ Marks, T.J.; Kolb, J.R. Chem. Rev. 1977, 77, 263.
- ↑ .
- ↑ Besora, M.; Lledós, A. "Coordination Modes and Hydride Exchange Dynamics in Transition Metal Tetrahydroborate Complexes" Structure and Bonding (2008) volume 130, p. 149–202. doi:10.1007/430_2007_076
- ↑ Franz, H.; Fusstetter, H.; Nöth. H. Z. Anorg. Allg. Chem. 1976, 427, 97–113.
- 1 2 3 4 Jensen, J. A.; Gozum, J. E.; Pollina, D. M.; Girolami, G. S. "Titanium, zirconium, and hafnium tetrahydroborates as "tailored" CVD precursors for metal diboride thin films" J. Am. Chem. Soc. 1988, 110, 1643–1644. doi:10.1021/ja00213a058
External links
| Wikiquote has quotations related to: Borohydride |