RAC3
| View/Edit Human | View/Edit Mouse |
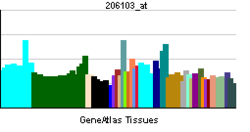
Ras-related C3 botulinum toxin substrate 3 is a protein that in humans is encoded by the RAC3 gene.[3]
The protein encoded by this gene is a GTPase that belongs to the RAS superfamily of small GTP-binding proteins. Members of this superfamily appear to regulate a diverse array of cellular events, including the control of cell growth, cytoskeletal reorganization, and the activation of protein kinases.[3]
Interactions
RAC3 has been shown to interact with CIB1[4] and HNF1A.[5]
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- 1 2 "Entrez Gene: RAC3 ras-related C3 botulinum toxin substrate 3 (rho family, small GTP binding protein Rac3)".
- ↑ Haataja, Leena; Kaartinen Vesa; Groffen John; Heisterkamp Nora (March 2002). "The small GTPase Rac3 interacts with the integrin-binding protein CIB and promotes integrin alpha(IIb)beta(3)-mediated adhesion and spreading". J. Biol. Chem. United States. 277 (10): 8321–8. doi:10.1074/jbc.M105363200. ISSN 0021-9258. PMID 11756406.
yes
- ↑ Soutoglou, E; Papafotiou G; Katrakili N; Talianidis I (April 2000). "Transcriptional activation by hepatocyte nuclear factor-1 requires synergism between multiple coactivator proteins". J. Biol. Chem. UNITED STATES. 275 (17): 12515–20. doi:10.1074/jbc.275.17.12515. ISSN 0021-9258. PMID 10777539.
yes
Further reading
- Didsbury J, Weber RF, Bokoch GM, et al. (1989). "rac, a novel ras-related family of proteins that are botulinum toxin substrates.". J. Biol. Chem. 264 (28): 16378–82. PMID 2674130.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery.". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Haataja L, Groffen J, Heisterkamp N (1997). "Characterization of RAC3, a novel member of the Rho family.". J. Biol. Chem. 272 (33): 20384–8. doi:10.1074/jbc.272.33.20384. PMID 9252344.
- Courjal F, Chuchana P, Theillet C, Fort P (1997). "Structure and chromosomal assignment to 22q12 and 17qter of the ras-related Rac2 and Rac3 human genes.". Genomics. 44 (2): 242–6. doi:10.1006/geno.1997.4871. PMID 9299243.
- Mira JP, Benard V, Groffen J, et al. (2000). "Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway". Proc. Natl. Acad. Sci. U.S.A. 97 (1): 185–9. doi:10.1073/pnas.97.1.185. PMC 26637
 . PMID 10618392.
. PMID 10618392. - Soutoglou E, Papafotiou G, Katrakili N, Talianidis I (2000). "Transcriptional activation by hepatocyte nuclear factor-1 requires synergism between multiple coactivator proteins". J. Biol. Chem. 275 (17): 12515–20. doi:10.1074/jbc.275.17.12515. PMID 10777539.
- Morris CM, Haataja L, McDonald M, et al. (2000). "The small GTPase RAC3 gene is located within chromosome band 17q25.3 outside and telomeric of a region commonly deleted in breast and ovarian tumours". Cytogenet. Cell Genet. 89 (1–2): 18–23. doi:10.1159/000015583. PMID 10894930.
- Gnanapragasam VJ, Leung HY, Pulimood AS, et al. (2002). "Expression of RAC 3, a steroid hormone receptor co-activator in prostate cancer". Br. J. Cancer. 85 (12): 1928–36. doi:10.1054/bjoc.2001.2179. PMC 2364015
 . PMID 11747336.
. PMID 11747336. - Haataja L, Kaartinen V, Groffen J, Heisterkamp N (2002). "The small GTPase Rac3 interacts with the integrin-binding protein CIB and promotes integrin alpha(IIb)beta(3)-mediated adhesion and spreading". J. Biol. Chem. 277 (10): 8321–8. doi:10.1074/jbc.M105363200. PMID 11756406.
- De Langhe S, Haataja L, Senadheera D, et al. (2002). "Interaction of the small GTPase Rac3 with NRBP, a protein with a kinase-homology domain". Int. J. Mol. Med. 9 (5): 451–9. doi:10.3892/ijmm.9.5.451. PMID 11956649.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241
 . PMID 12477932.
. PMID 12477932. - Zhang A, Yeung PL, Li CW, et al. (2004). "Identification of a novel family of ankyrin repeats containing cofactors for p160 nuclear receptor coactivators". J. Biol. Chem. 279 (32): 33799–805. doi:10.1074/jbc.M403997200. PMID 15184363.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The Status, Quality, and Expansion of the NIH Full-Length cDNA Project: The Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928
 . PMID 15489334.
. PMID 15489334. - Hwang SL, Chang JH, Cheng TS, et al. (2006). "Expression of Rac3 in human brain tumors". Journal of Clinical Neuroscience. 12 (5): 571–4. doi:10.1016/j.jocn.2004.08.013. PMID 15993075.
- Chan AY, Coniglio SJ, Chuang YY, et al. (2005). "Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion". Oncogene. 24 (53): 7821–9. doi:10.1038/sj.onc.1208909. PMID 16027728.
- Rual JF, Venkatesan K, Hao T, et al. (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. doi:10.1038/nature04209. PMID 16189514.
- Baugher PJ, Krishnamoorthy L, Price JE, Dharmawardhane SF (2006). "Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phenotype of human breast cancer cells". Breast Cancer Res. 7 (6): R965–74. doi:10.1186/bcr1329. PMC 1410764
 . PMID 16280046.
. PMID 16280046. - Watabe-Uchida M, John KA, Janas JA, et al. (2006). "The Rac activator DOCK7 regulates neuronal polarity through local phosphorylation of stathmin/Op18". Neuron. 51 (6): 727–39. doi:10.1016/j.neuron.2006.07.020. PMID 16982419.
- Hajdo-Milasinović A, Ellenbroek SI, van Es S, et al. (2007). "Rac1 and Rac3 have opposing functions in cell adhesion and differentiation of neuronal cells". J. Cell. Sci. 120 (Pt 4): 555–66. doi:10.1242/jcs.03364. PMID 17244648.
External links
- RAC3 Info with links in the Cell Migration Gateway
This article is issued from Wikipedia - version of the 6/5/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.