Silver subfluoride
 | |
| Names | |
|---|---|
| IUPAC name
silver(0,I) fluoride | |
| Identifiers | |
| 1302-01-8 | |
| Properties | |
| Ag2F | |
| Molar mass | 234.734 g/mol |
| Appearance | Bronze-colored crystals with green luster |
| Density | 8.6 g/cm3, solid |
| Melting point | 90 °C (194 °F; 363 K) decomposition |
| reacts | |
| Related compounds | |
| Related compounds |
Silver(I) fluoride Silver(II) fluoride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Silver Subfluoride is the inorganic compound with the formula Ag2F. This is an unusual example of a compound where the oxidation state of silver is fractional. The compound is produced by the reaction of silver and silver(I) fluoride:[1]
- Ag + AgF → Ag2F
It forms small crystals with a bronze reflex and is a good conductor of electricity. On contact with water almost instant hydrolysis occurs with the precipitation of silver (Ag) powder.
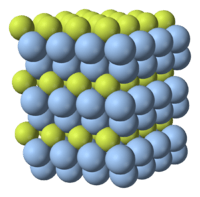
Crystal structure
Ag2F adopts the anti-CdI2 crystal structure, i.e. the same structure as cadmium iodide, CdI2, but with "Ag½+ " centres in the I− positions and F− in the Cd2+ positions.[2] The shortest distance between silver atoms is 299.6 pm (compared to 289 pm in the metal).[3]
References
- ↑ Lee Poyer, Maurice Fielder, Hugh Harrison, Burl E. Bryant "Disilver Fluoride: (Silver “Subfluoride”)" Inorganic Syntheses, 1957, Volume 5, 92–94. doi:10.1002/9780470132364.ch6
- ↑ A Williams (April 1989). "Neutron powder diffraction study of silver subfluoride". J. Phys.: Condens. Matter. 1 (15): 2569–2574. doi:10.1088/0953-8984/1/15/002.
- ↑ Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
This article is issued from Wikipedia - version of the 6/11/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.