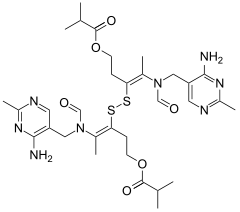
Sulbutiamine
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | A11DA02 (WHO) |
| Pharmacokinetic data | |
| Biological half-life | 5 hours |
| Excretion | Renal |
| Identifiers | |
| |
| Synonyms | Arcalion, bisibuthiamine, enerion, youvitan |
| CAS Number |
3286-46-2 |
| PubChem (CID) | 71124 |
| ChemSpider |
16736830 |
| UNII |
42NCM1BW43 |
| KEGG |
D01319 |
| ECHA InfoCard | 100.019.944 |
| Chemical and physical data | |
| Formula | C32H46N8O6S2 |
| Molar mass | 702.89 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Sulbutiamine (brand name: Arcalion) is a synthetic derivative of thiamine (vitamin B1). As a dimer of two modified thiamine molecules, it is a lipophilic compound that crosses the blood–brain barrier more readily than thiamine and increases the levels of thiamine and thiamine phosphate esters in the brain.[1] Sulbutiamine was discovered in Japan in an effort to develop more useful thiamine derivatives since it was hoped that increasing the lipophilicity of thiamine would result in better pharmacokinetic properties.[2]
Although its clinical efficacy is uncertain,[3] it is the only compound used to treat asthenia that is known to selectively target the areas that are involved in the condition.[4] In addition to its use as a treatment for chronic fatigue, sulbutiamine may improve memory, reduce psycho-behavioural inhibition, and improve erectile dysfunction. At therapeutic dosages, it has few reported adverse effects. It is available for over-the-counter sale as a nutritional supplement.
History

The history of sulbutiamine is closely tied to the study of thiamine in Japan. A deficiency of thiamine causes a nervous system disorder called beriberi.[5] Until the twentieth century, beriberi was prevalent in Japan and other Asian countries due to the widespread dependence on white rice as a staple food. The relationship between beriberi and diet was first noted by a navy surgeon named Takaki Kanehiro.[6] Additional work resulted in the discovery of thiamine, which was isolated in 1926 and synthesized in 1936. The establishment of a Vitamin B Research Committee in Japan led to additional scientific investigation into the properties of thiamine and its derivatives.[6]
The first lipophilic thiamine derivative to be discovered was allithiamine, which was isolated from garlic (Allium sativum) in 1951.[7] Allithiamine is an allyl disulfide derivative. After the discovery of allithiamine, several additional derivatives were synthesized with the hope that they would have better pharmacokinetic properties than thiamine. Thiamine is unable to diffuse across plasma membranes because it has a positively charged thiazole moiety. Instead, it must be transported across plasma membranes by high affinity carriers, and the rate of transport is low.[8] Sulbutiamine overcomes the poor oral bioavailability of thiamine because it is highly lipophilic. The synthesis of sulbutiamine was reported by Taisho Pharmaceutical Co. in 1965.[9]
Therapeutic uses
Sulbutiamine is indicated for the treatment of asthenia. Asthenia is a condition of chronic fatigue that is cerebral rather than neuromuscular in origin.[10]
Availability
Sulbutiamine is available in several forms. Arcalion is supplied in 200 mg tablets,[11] and generic sulbutiamine is supplied in tablets, capsules, and powder. The manufacturer of Arcalion recommends no more than 600 mg per day.[11]
Adverse effects
Sulbutiamine has few reported adverse effects at therapeutic dosages. According to the manufacturer of Arcalion, a mild skin allergy may occur, and mild agitation has also been observed in elderly patients.[11]
See also
References
- ↑ Bettendorff L, Weekers L, Wins P, Schoffeniels E (1990). "Injection of sulbutiamine induces an increase in thiamine triphosphate in rat tissues". Biochem Pharmacol. 40 (11): 2557–60. doi:10.1016/0006-2952(90)90099-7. PMID 2268373.
- ↑ Volvert ML, Seyen S, Piette M, Evrard B, Gangolf M, Plumier JC, Bettendorff L (2008). "Benfotiamine, a synthetic S-acyl thiamine derivative, has different mechanisms of action and a different pharmacological profile than lipid-soluble thiamine disulfide derivatives". BMC Pharmaco. 8: 10. doi:10.1186/1471-2210-8-10. PMC 2435522
 . PMID 18549472.
. PMID 18549472. - ↑ Tiev KP, Cabane J, Imbert JC (1999). "[Treatment of chronic postinfectious fatigue: randomized double-blind study of two doses of sulbutiamine (400-600 mg/day) versus placebo]". Rev Med Interne. 20 (10): 912–8. PMID 10573727.
- ↑ Van Reeth O (1999). "Pharmacologic and therapeutic features of sulbutiamine". Drugs Today (Barc). 35 (3): 187–92. PMID 12973384.
- ↑ Inouye K, Katsura E. "Etiology and pathology of beriberi." In: Shimazono N, Katsura E, editors. Beriberi and Thiamine Igaku Shoin Ltd (1965) p. 1–28
- 1 2 Lonsdale D (2006). "A Review of the Biochemistry, Metabolism and Clinical Benefits of Thiamin(e) and Its Derivatives". Evid Based Complement Alternat Med. 3 (1): 49–59. doi:10.1093/ecam/nek009. PMC 1375232
 . PMID 16550223.
. PMID 16550223. - ↑ Lonsdale D (2004). "Thiamine tetrahydrofurfuryl disulfide: a little known therapeutic agent". Med Sci Monit. 10 (9): 199–203. PMID 15328496.
- ↑ Bettendorff L, Wins P (1994). "Mechanism of thiamine transport in neuroblastoma cells. Inhibition of a high affinity carrier by sodium channel activators and dependence of thiamine uptake on membrane potential and intracellular ATP". J Biol Chem. 269 (20): 14379–85. PMID 8182042.
- ↑ Ammo T, Sakai T, Fujihira EL, Aizawa T (1965). "Thiamine disulfide derivatives. I. Syntheses of thiamine disulfide derivatives". Bitamin. 32 (2): 260–4.; Ammo T, Sakai T, Aizawa T, Fujihira E, Naganuma A (1965). "Thiamine disulfide derivatives. II. Biological activities of thiamine disulfide derivatives". Bitamin. 32 (2): 265–77.; DE granted 1620538, Ammo T, Sakai T, Fujihira E, Aizawa T, "Vitamin-B1-Derivat, Verfahren zu dessen Herstellung und dessen Verwendung [Vitamin B1 derivative, process for its preparation and its use]", published 1969-10-16, issued 1977-12-31, assigned to Taisho Pharma Co Ltd. DE1620538 in turn claims a priority date of 1965-03-18 from the Japanese patent application JP19650015344.
- ↑ Layzer RB (1998). "Asthenia and the chronic fatigue syndrome". Muscle Nerve. 21 (12): 1609–11. doi:10.1002/(SICI)1097-4598(199812)21:12<1609::AID-MUS1>3.0.CO;2-K. PMID 9843061.
- 1 2 3 "Serdia Pharmaceuticals Arcalion summary". Retrieved 1 August 2012.