Technetium (99mTc) tilmanocept
 | |
| Clinical data | |
|---|---|
| Trade names | Lymphoseek |
| AHFS/Drugs.com | lymphoseek |
| Pregnancy category |
|
| Routes of administration | Intradermal, subcutaneous |
| ATC code | V09IA09 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 1.75 to 3.05 hours at injection site |
| Identifiers | |
| |
| Chemical and physical data | |
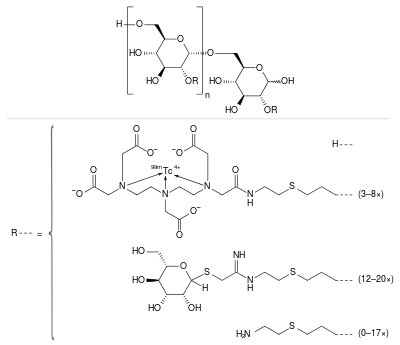
| Formula | (C6H10O5)n(C19H28N4O9S99mTc)3–8(C13H24N2O5S2)12–20(C5H11NS)0–17 |
| Molar mass | 15,281–23,454 g/mol[1] |
Technetium (99mTc) tilmanocept, trade name Lymphoseek, is a radiopharmaceutical diagnostic imaging agent approved by the U.S. Food and Drug Administration (FDA) for the imaging of lymph nodes.[1][2] It is used to locate those lymph nodes which may be draining from tumors, and assist doctors in locating those lymph nodes for removal during surgery.[3]
References
- 1 2 FDA Professional Drug Information
- ↑ http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm343525.htm
- ↑ Marcinow, A. M.; Hall, N.; Byrum, E.; Teknos, T. N.; Old, M. O.; Agrawal, A. (2013). "Use of a novel receptor-targeted (CD206) radiotracer, 99mTc-tilmanocept, and SPECT/CT for sentinel lymph node detection in oral cavity squamous cell carcinoma: Initial institutional report in an ongoing phase 3 study". JAMA otolaryngology-- head & neck surgery. 139 (9): 895–902. doi:10.1001/jamaoto.2013.4239. PMID 24051744.
This article is issued from Wikipedia - version of the 8/28/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
