Titanium(III) fluoride
 | |
 | |
| Names | |
|---|---|
| IUPAC name
trifluorotitanium | |
| Other names
Titanium trifluoride, Titanous fluoride, titanium fluoride, titanium fluorideminpurplebrownpowder, trifluorotitanium | |
| Identifiers | |
| 13470-08-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 75341 |
| ECHA InfoCard | 100.033.379 |
| EC Number | 236-732-4 |
| PubChem | 83506 |
| |
| |
| Properties | |
| TiF3 | |
| Molar mass | 104.862 g/mol |
| Appearance | violet to purple-red powder |
| Density | 3.4 g/cm3 |
| Melting point | 1,200 °C (2,190 °F; 1,470 K) |
| Boiling point | 1,400 °C (2,550 °F; 1,670 K) |
| sparingly soluble | |
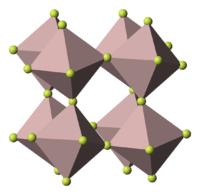
| Structure | |
| Rhombohedral, hR24 | |
| R-3c, No. 167 | |
| Hazards | |
| EU classification (DSD) |
not listed |
| Related compounds | |
| Related compounds |
Titanium difluoride Titanium trichloride Titanium tribromide Titanium triiodide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Titanium(III) fluoride (TiF3) is a chemical compound of titanium in its oxidation state +3. It can be obtained by the reaction of titanium(III) oxide with hydrofluoric acid (this reaction reverses if TiF3 is dissolved in excess water), or by reduction of titanium tetrafluoride.
Conditions/substances to avoid are: strong acids, strong oxidizers.
References
This article is issued from Wikipedia - version of the 11/16/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.