Vonoprazan
 | |
| Clinical data | |
|---|---|
| Trade names | Takecab |
| ATC code | None |
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | 80% |
| Metabolism | Hepatic, by cytochrome P450 (3A4, 2B6, 2C19, 2D6) |
| Biological half-life | 7.7 h |
| Duration of action | > 24 h |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | 881681-00-1 1260141-27-2 (fumarate) |
| ChemSpider | 13112797 |
| Chemical and physical data | |
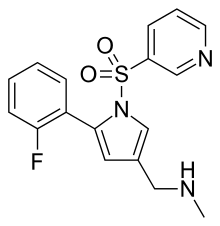
| Formula | C17H16FN3O2S |
| Molar mass | 345.39 g·mol−1 |
| 3D model (Jmol) | Interactive image |
| |
| |
Vonoprazan fumarate is a first-in-class potassium-competitive acid blocker. It was approved in the Japanese market in February 2015.[1]
Vonoprazan can be used for the treatment of gastroduodenal ulcer (including some drug-induced peptic ulcers) and reflux esophagitis, and can be combined with antibiotics for the eradication of Helicobacter pylori.[2]
References
- ↑ Garnock-Jones KP (2015). "Vonoprazan: first global approval". Drugs. 75 (4): 439–43. doi:10.1007/s40265-015-0368-z. PMID 25744862.
- ↑ Echizen H (2016). "The First-in-Class Potassium-Competitive Acid Blocker, Vonoprazan Fumarate: Pharmacokinetic and Pharmacodynamic Considerations". Clin Pharmacokinet. 55 (4): 409–18. doi:10.1007/s40262-015-0326-7. PMID 26369775.
This article is issued from Wikipedia - version of the 10/29/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.