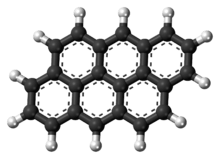
Anthanthrene
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Dibenzo[def,mno]chrysene | |
| Other names
Anthanthren; Dibenzo[cd,jk]pyrene | |
| Identifiers | |
| 191-26-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 8764 |
| ECHA InfoCard | 100.005.351 |
| KEGG | C19327 |
| |
| |
| Properties | |
| C22H12 | |
| Molar mass | 276.33 g/mol |
| Appearance | Golden yellow solid |
| Melting point | 261 °C (502 °F; 534 K) |
| Insoluble | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Anthanthrene is a polycyclic aromatic hydrocarbon.[1] According to the International Agency for Research on Cancer, as of 2006 there was "limited evidence in experimental animals" that it is a carcinogen.[2]
References
- ↑ Clar, E. (1964). Polycyclic Hydrocarbons. New York: Academic Press.
- ↑ "PAHs: IARC Working Group, 2006". Carcinogenic Risk In Occupational Settings.
This article is issued from Wikipedia - version of the 7/7/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.