Ethylene dione
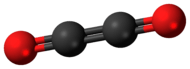 | |
| Names | |
|---|---|
| IUPAC name
Ethene-1,2-dione | |
| Systematic IUPAC name
Ethenedione | |
| Other names
Dicarbon dioxide Dimeric carbon monoxide | |
| Identifiers | |
| 4363-38-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 278619 |
| PubChem | 314937 |
| |
| |
| Properties | |
| C2O2 | |
| Molar mass | 56.02 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethylene dione or ethylenedione, also called dicarbon dioxide, ethenedione, or ethene-1,2-dione, is the name given to a chemical compound with the formula C2O2 or OCCO. It is a dimer of carbon monoxide and belongs to the oxocarbon series. Because it is a dimer, it shares an empirical formula with CO. It can be thought of as ketene of glyoxylic acid (OHCCOOH).
The existence of OCCO was first suggested in 1913.[1] However, despite its deceptively "simple" structure, for over a century the compound had eluded all attempts to synthesize and observe it. Such elusive nature had earned OCCO the reputation of a hypothetical compound and a mysterious, "exceedingly coy molecule".[2]
It was not until 2015 that a group of chemists from the University of Arizona in Tucson (United States) reported the first spectroscopic characterization of OCCO, confirming its existence as a transient molecule.[3] The Arizona group created OCCO using laser light to eject electrons from the corresponding stable singly-charged anions.[4][5]
Despite the existence of the closed-shell Kekulé structure, O=C=C=O, the lowest bound state of ethyledione is a triplet. Therefore, bound OCCO is formally a diradical, with an electronic structure motif similar to the oxygen molecule. However, when the molecule is distorted away from its equilibrium geometry, the potential surfaces of the triplet and singlet states intersect, allowing for intersystem crossing to the singlet state, which is unbound and dissociates to two ground-state CO molecules. The timescale of the intersystem crossing was predicted to be 0.5 ns,[6] making triplet OCCO a transient, yet spectroscopically long-lived molecule.
On the other hand, the monoanion of ethylenedione, OCCO−, as well as the divalent anion C
2O2−
2, called acetylenediolate, are both stable.
Koch's glyoxylide
In the 1940s, Detroit physician William Frederick Koch claimed that he had synthesized this compound, which he called glyoxylide, and that it was an antidote to the toxins that caused a long list of ailments, including diabetes and cancer. The claims were false and the drug was classified as a fraud by the FDA.[7]
See also
- Cyclohexanehexone C6O6, also called triquinoyl, formally a trimer of ethylene dione.
References
- ↑ H. Staudinger, E. Anthes, Ber. Dtsch. Chem. Ges. 1913, 46, 1426.
- ↑ Lewars, Errol (2008), "9 – Ethenedione C2O2", Modeling Marvels, Springer
- ↑ A. R. Dixon, T. Xue and A. Sanov, "Spectroscopy of Ethylenedione", Angew. Chem. Int. Ed., 54, 8764-8767 (2015), doi:10.1002/anie.201503423.
- ↑ "UA Researchers Reveal Elusive Molecule". UA News. 13 July 2015. Retrieved 14 July 2015.
- ↑ "An elusive molecule—finally revealed". Phys.Org. 13 July 2015. Retrieved 14 July 2015.
- ↑ D. Schröder, C. Heinemann, H. Schwarz, J. N. Harvey, S. Dua, S. J. Blanksby, and John, H. Bowie, "Ethylenedione: An Intrinsically Short-Lived Molecule", Chem. Eur. J., 4, 2550-2557 (1998).
- ↑ William W. Goodrich interview for FDA Oral History Program, Part 2. Rockville, Maryland, 15 October 1986.