Serine dehydratase
| Serine dehydratase | |
|---|---|
 | |
| Identifiers | |
| Symbol | SDS |
| Entrez | 10993 |
| HUGO | 10691 |
| OMIM | 182128 |
| RefSeq | NM_006843 |
| UniProt | P20132 |
| Other data | |
| EC number | 4.3.1.17 |
| Locus | Chr. 12 q24.21 |
Serine dehydratase or L-serine ammonia lyase (SDH) is in the β-family of pyridoxal phosphate-dependent (PLP) enzymes. SDH is found widely in nature, but its structural and chemical properties vary greatly among species. SDH is found in yeast, bacteria, and the cytoplasm of mammalian hepatocytes. The reaction it catalyzes is the deamination of L-serine to yield pyruvate, with the release of ammonia.[1]
This enzyme has 1 substrate, L-serine, and two products, pyruvate and NH3, and uses 1 cofactor, pyridoxal phosphate (PLP). The enzyme's main role is in gluconeogenesis in the liver's cytoplasm. By orienting the substrates and utilizing the PLP coenzyme, SDH lowers the activation energy to convert L-Serine into pyruvate, which can then be converted into glucose.
Nomenclature
Serine Dehydratase is also known as:[2]
- L-serine ammonia-lyase
- Serine deaminase
- L-hydroxyaminoacid dehydratase
- L-serine deaminase
- L-serine dehydratase
- L-serine hydro-lyase
Enzyme structure
HoloEnzyme: The holoenzyme SDH contains 319 residues, 1 PLP cofactor molecule, and 131 water molecules.[1] The overall fold of the monomer is very similar to that of other PLP-dependent enzymes of the Beta-family. The enzyme contains a large domain (catalytic domain or PLP- binding domain) and a small domain. The domains are joined by two peptide linkers (residues 32-35 and 138-146), with the internal gap created being the space for the active site[1] (Figure 1).
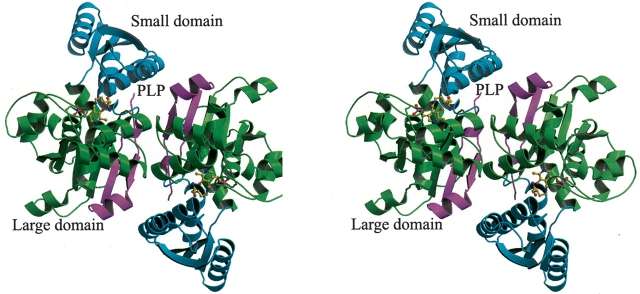
Figure 1 shows the large catalytic domain in purple and cyan and the small regulatory domain in green in a monomer of Serine Dehydratase. Two monomers(left and right) are shown and the coenzyme PLP is placed in the crevice between the two domains. [1]
Two Dimers: Two monomers of hSDS (human SDH) come together to make a dimer. The interface between the two monomers is formed through hydrogen bonds and hydrophobic interactions. The monomer–monomer contacts involve six pairs of hydrogen bonds formed between 10 residues (Arg98-Asn260, Leu310-Asn260, and Leu265-Lys263). Additional interactions include a number of hydrophobic contacts between the residues Met17, Lys21, Asn101, Glu102, Ser306, Ile308, Ser309, and Ile264 in each monomer.[1] (Figure 2).
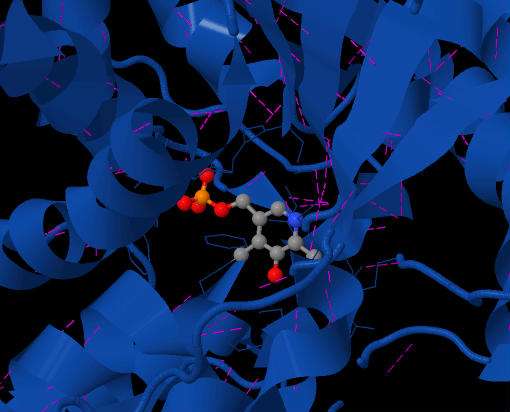
Figure 2 shows the PLP coenzyme situated in the active site of SDH. The purple dashes are the hydrogen bonds involved. Top view of the enzyme.
Cofactor Binding Site: The PLP cofactor is positioned in between the Beta-strands 7 and 10 of the large domain and lies on the large internal gap made between small and large domain. The cofactor is covalently bonded through a Schiff base linkage to Lys41. The cofactor is sandwiched between the side chain of Phe40 and the main chain of Ala222. Each of the polar substituents of PLP is coordinated by functional groups: the pyridinium nitrogen of PLP is hydrogen-bonded to the side chain of Cys303, the C3-hydroxyl group of PLP is hydrogen-bonded to the side chain of Asn67, and the phosphate group of PLP is coordinated by main chain amides from the tetraglycine loop.[1][3] (Figure 3 and Figure 4).
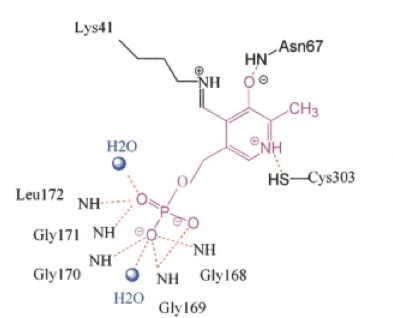
Figure 3 shows the hydrogen bonding in the active site of SDH. Hydrogen bonds are (red) between protein, water (blue balls) and the cofactor PLP (purple).[1]
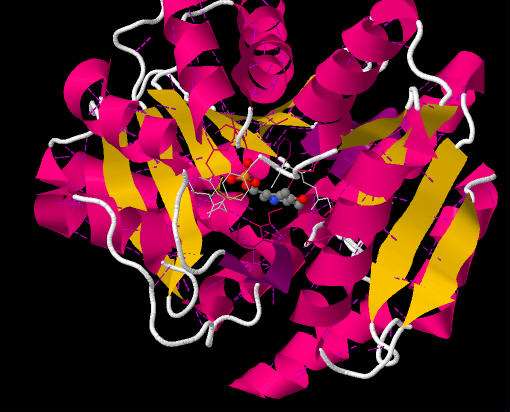
Figure 4 shows the alpha helices (pink) and beta sheets (yellow) involved in the secondary structure of SDH.
Enzyme mechanism
The degradation of serine to pyruvate is an example of a pyridoxal phosphate-dependent (PLP) catalyzed Beta-elimination reaction. Beta-eliminations[4] mediated by PLP yields products that have undergone a two-electron oxidation at C-alpha. In general, beta-eliminations involve the removal of a halide and a proton from the adjacent beta-carbon to give a double bond; thus, the origin of the double bond pi electrons are from the C-H bond on the beta carbon of the substrate.
Beta eliminations occur with no net oxidation or reduction of PLP. In overall terms, the reaction catalyzed by serine dehydratase involves two steps: catalytic elimination and a nonenzymatic hydrolysis reaction. The main role of SDH is to lower the activation energy of this reaction by binding the coenzyme and substrate in a particular conformational geometry.
Mechanistic Steps:[5]
(In panel 1 of Figure 5) In the SDH enzyme’s active site, Lys41 is located above the PLP molecule with its R group NH2 connected to C4 of PLP by a Schiff base linkage. The phosphate group of PLP is located in a pocket of G residues. Serine enters the active site and its positively charged amino group attracts the negatively charged phosphate group of PLP. The intermediate PLP-Ser aldimine is created. SDH's role is to orient the serine molecule’s Calpha-H parallel to the overlapping 2p orbitals of the PLP pi system; in other words, SDH holds serine perpendicular to the plane of the PLP ring.[5] ( See Figure 6 for orientation of substrate with PLP).
(In panel 2 of Figure 5) The amino group of serine protonates the PLP phosphate by forming a H-bond. The deprotonated amino group of Serine is now a good nucleophile that attacks the Lys-PLP Schiff base at the C4 carbon (shown in panel 1). The Lys41 is released from PLP.[5]
(In panel 3 of Figure 5) The COOH group of serine is positioned tightly in the SDH enzyme so that the serine molecule is perpendicular to the PLP pi system. The R group OH group participates in two hydrogen bonds with SDH’s Ala222 and the protonated phosphate of PLP. The protonated phosphate of PLP then acts as an acid and donates its proton to hydroxyl of serine. In a concerted fashion, the R group hydrogen of serine is removed by Lys41 and water is released. The intermediate created is PLP-aminoacrylate.[5]
In the reaction as the water leaves from the Beta-carbon of the substrate, the SDH orients the newly created double bond perpendicular to the PLP plane (Figure 6). This allows the new pi bonds between Calpha and Cbeta to form resonance with the PLP pi system.[5] (Figure 6)
(In panel 4 of Figure 5) Lys41 from SDH's active site attacks C4 of PLP, forming a tetrahedral intermediate.[5]
(In panel 5 of Figure 5) A Schiff base linkage is then made and the aminoacrylate group is released as pyruvate.[5]
(In panel 6 of Figure 5) The aminoacrylate released from PLP is unstable in aqueous solution and rapidly tautomerizes to the preferred imine form; this is spontaneously hydrolyzed to yield alpha-keto acid product of pyruvate. The aminoacrylate is nonezymatically deaminated to pyruvate by hydrolysis. The enzyme-PLP Schiff base linkage is reformed.[5]
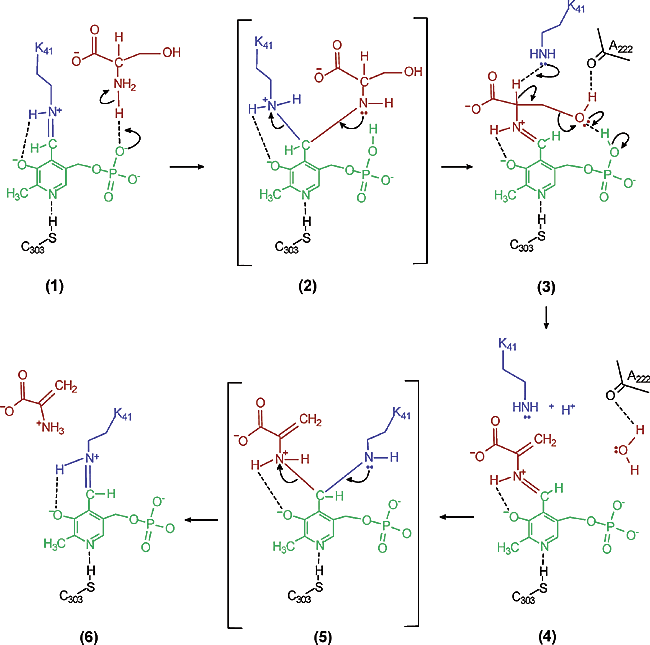
Figure 5 shows the mechanism of converting L-Serine into Pyruvate. The Figure displays the SDH active site, PLP coenzyme and substrate.[1]
Figure 6 shows the role of SDH in orienting the PLP molecule perpendicular to the substrate Serine.
Inhibitors
According to the series of assays performed by Cleland (1967), the linear rate of pyruvate formation at various concentrations of inhibitors demonstrated that L-cysteine and D-serine competitively inhibit the enzyme SDH.[6] The reason that SDH activity is inhibited by L-cysteine is because an inorganic sulfur is created from L-Cysteine via Cystine Desulfrase and sulfur-containing groups are known to promote inhibition.[7] In addition, researchers have shown that L-threonine competitively inhibits Serine Dehydratase as well.
Moreover, insulin is known to accelerate glycolysis and repress induction of liver serine dehydratase in adult diabetic rats.[8] Studies have been conducted to show insulin causes a 40-50% inhibition of the induction serine dehydratase by glucagon in hepatocytes of rats.[9] Studies have also shown that insulin and epinephrine inhibit Serine Dehydratase activity by inhibiting transcription of the SDH gene in the hepatocytes.[10] Similarly, increasing levels of glucagon, increase the activity of SDH because this hormone up-regulates the SDH enzyme. This makes sense in the context of gluconeogenesis. The main role of SDH is to create pyruvate that can be converted into free glucose. And glucagon gives the signal to repress gluconeogenesis and increase the amount of free glucose in the blood by releasing glycogen stores from the liver.
Homocysteine, a compound that SDH combines with Serine to create cystathionine, also noncompetitively inhibits the action of SDH. Studies have shown that homocysteine reacts with SDH's PLP coenzyme to create a complex. This complex is devoid of coenzyme activity and SDH is not able to function (See Enzyme Mechanism Section).[11] In general, homocysteine is an amino acid and metabolite of methionine; increased levels of homocysteine can lead to homocystinuria(see section Disease Relevance).[12]
Biological function
In general, SDH levels decrease with increasing mammalian size.[13] In fact, mammals catabolize serine into pyruvate with the enzymes serine hydroxymethyltransferase and glycine cleavage enzymes rather than SDH.
Studies show that the SDH enzyme from rat hepatocytes plays an important role in gluconeogenesis; its activity is augmented by high-protein diets and starvation. During periods of low carbohydrates, serine is converted into pyruvate via SDH. This pyruvate enters the mitochondria where it can be converted into oxaloacetate, and, thus, glucose.[14]
Figure 7 shows the possible routes of L-Serine conversion into glucose during gluconeogenesis.
However, interestingly, little is known about the properties and the function of human SDH because human liver has low SDH activity. In a study done by Yoshida and Kikuchi, routes of glycine breakdown were measured. Glycine can be converted into serine and either become pyruvate via serine dehydratase or undergo oxidative cleavage into methylene-THF, ammonia, and carbon dioxide. Results showed the secondary importance of the SDH pathway.[14][15]
Disease relevance
Although there is much controversy over the role of SDH in human hepatocytes, studies have shown that nonketotic hyperglycemia is due to the deficiency of threonine dehydratase, a close corollary to serine dehydratase. Serine dehydratase has also been found to be absent in human colon carcinoma and rat sarcoma. The observed enzyme imbalance in these tumors shows that an increased capacity for the synthesis of serine is coupled to its utilization for nucleotide biosynthesis as a part of the biochemical commitment to cellular replication in cancer cells. This pattern is found in sarcomas and carcinomas, and in tumors of human and rodent origin Thus, SDH is significant in the development of hyperglycemia and tumors.[16]
In addition, homocystinuria is a hereditary disease caused by the deficiency of L-serine dehydratase. Its symptoms include mental retardation, death, atherosclerosis, and coronary thrombosis as well as dislocation of the eye lens. Homocystinuria is a disease characterized by high urine and plasma levels of homocysteine. L-Serine dehydratase condenses homocysteine with serine to form cystathionine.[17] The foregoing paragraph is however mistaken as the paragraph equates SDS with CBS which was once thought to be the case but which is now known not to be the case.
Evolution
Comparing human and rat serine dehydratase using a cDNA library was identical except for a 36 amino acid residue stretch. The overall homology between rat SDH and human SDH is 81% in the nucleotide sequence and 84% in the amino acid sequence. Similarities have also been shown between yeast and E. coli threonine dehydratase and human serine dehydratase. Human SDH shows sequence homology of 27% with the yeast enzyme and 27% with the E. coli enzyme.[18]
Additionally, the primary structures are shown to be similar between mammalian SDH and microbial threonine dehydratase, especially in the sequences surrounding the PLP cofactor and the G-residues surrounding the PLP’s phosphate group. Thus, in PLP enzymes, there is high conservation of the active site residues during evolution. With active site sequence conservation, it is suggested that dehydratase enzymes originated from a common ancestor.[18]
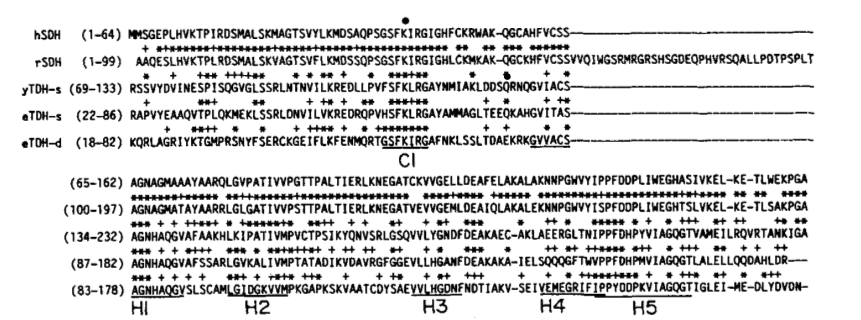
Figure 8 shows the sequence similarities of the amino acid sequence of human SDH with those of rat SDH, and yeast and E. coli threonine dehydratases. Asterisks and crosses represent sequence similarity to human SDH.[18]
In an analysis done by Mehta and Christen from the Center for Bioinformatics and Biotechnology, the pyridoxal-5-phosphate (vitamin B6)-dependent enzymes that act on amino acid substrates have multiple evolutionary origins. The overall B6 enzymes diverged into four independent evolutionary lines: α family (i.e. aspartate aminotransferase), β family (serine dehydratase),D-alanine aminotransferase family and the alanine racemase family. An example of the evolutionary similarity in the Beta family is seen in the mechanism. The β enzymes are all lyases and catalyze reactions where Cα and Cβ participate. Overall, in the PLP-dependent enzymes, the PLP in every case is covalently attached via an imine bond to the amino group in the active site.[19]
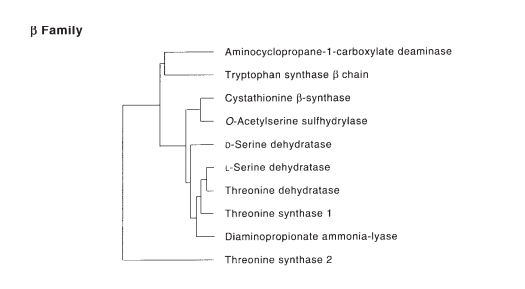
Figure 9 displays the evolutionary lineage of enzymes from PLP-dependent enzyme to the Beta family to SDH.
External links
- Serine dehydratase at the US National Library of Medicine Medical Subject Headings (MeSH)
References
- 1 2 3 4 5 6 7 8 Sun L, Bartlam M, Liu Y, Pang H, Rao Z (2005). "Crystal structure of the pyridoxal-5'-phosphate-dependent serine dehydratase from human liver". Protein Science. 14 (3): 791–8. doi:10.1110/ps.041179105. PMC 2279282
 . PMID 15689518.
. PMID 15689518. - ↑ "KEGG ENZYME Database Entry". Kyoto Encyclopedia of Genes and Genomes. Kanehisa Laboratories. Retrieved 17 May 2011.
- ↑ Toyota CG, Berthold CL, Gruez A, Jonsson S, Lindqvist Y, Cambillau C, Richards N (2008). "Differential Substrate Specificity and Kinetic Behavior of Escherichia coli YfdW and Oxalobacter formigenes Formyl Coenzyme a Transferase". Journal of Bacteriology. 190 (7): 2556–64. doi:10.1128/JB.01823-07. PMC 2293189
 . PMID 18245280.
. PMID 18245280. - ↑ http://chemwiki.ucdavis.edu/Organic_Chemistry/Organic_Chemistry_With_a_Biological_Emphasis/Chapter_14%3a_Reactions_with_stabilized_carbanion_intermediates_II/Section_14.4%3a_Pyridoxal_phosphate_-_an_electron_sink_cofactor[]
- 1 2 3 4 5 6 7 8 Yamada, Taro; Komoto, Junichi; Takata, Yoshimi; Ogawa, Hirofumi; Pitot, Henry C.; Takusagawa, Fusao (2003). "Crystal Structure of Serine Dehydratase from Rat Liver". Biochemistry. 42 (44): 12854–65. doi:10.1021/bi035324p. PMID 14596599.
- ↑ Gannon F, Bridgeland ES, Jones KM (1977). "L-serine dehydratase from Arthrobacter globiformis". The Biochemical Journal. 161 (2): 345–55. doi:10.1042/bj1610345. PMC 1164512
 . PMID 322657.
. PMID 322657. - ↑ Nakagawa H, Kimura H (1969). "The properties of crystalline serine dehydratase of rat liver". Journal of Biochemistry. 66 (5): 669–83. PMID 5358627.
- ↑ Freedland RA, Taylor AR (1964). "Studies on glucose-6-phosphatase and glutaminase in rat liver and kidney". Biochimica et Biophysica Acta. 92 (3): 567–71. doi:10.1016/0926-6569(64)90016-1. PMID 14264889.
- ↑ Miura S, Nakagawa H (1970). "Studies on the molecular basis of development of serine dehydratase in rat liver". Journal of Biochemistry. 68 (4): 543–8. PMID 5488777.
- ↑ Kanamoto, Ryuhei; Su, Yeu; Pitot, Henry C. (1991). "Effects of glucose, insulin, and cAMP on transcription of the serine dehydratase gene in rat liver". Archives of Biochemistry and Biophysics. 288 (2): 562–6. doi:10.1016/0003-9861(91)90236-C. PMID 1654838.
- ↑ Pestaña, Angel; Sandoval, Ignacio V.; Sols, Alberto (1971). "Inhibition by homocysteine of serine dehydratase and other pyridoxal 5′-phosphate enzymes of the rat through cofactor blockage". Archives of Biochemistry and Biophysics. 146 (2): 373–9. doi:10.1016/0003-9861(71)90139-1. PMID 4398884.
- ↑ Hurd, Russell W.; Hammond, Edward J.; Wilder, B.J. (1981). "Homocysteine induced convulsions: Enhancement by vitamin B6 and inhibition by hydrazine". Brain Research. 209 (1): 250–4. doi:10.1016/0006-8993(81)91190-2. PMID 6260308.
- ↑ Rowsell EV, Carnie JA, Wahbi SD, Al-Tai AH, Rowsell KV (1979). "L-serine dehydratase and l-serine-pyruvate aminotransferase activities in different animal species". Comparative Biochemistry and Physiology B. 63 (4): 543–55. doi:10.1016/0305-0491(79)90061-0. PMID 318433.
- 1 2 Snell, Keith (1984). "Enzymes of serine metabolism in normal, developing and neoplastic rat tissues". Advances in Enzyme Regulation. 22: 325–400. doi:10.1016/0065-2571(84)90021-9. PMID 6089514.
- ↑ Koyata H, Hiraga K (1991). "The glycine cleavage system: Structure of a cDNA encoding human H-protein, and partial characterization of its gene in patients with hyperglycinemias". American Journal of Human Genetics. 48 (2): 351–61. PMC 1683031
 . PMID 1671321.
. PMID 1671321. - ↑ Snell K, Natsumeda Y, Eble JN, Glover JL, Weber G (1988). "Enzymic imbalance in serine metabolism in human colon carcinoma and rat sarcoma". British Journal of Cancer. 57 (1): 87–90. doi:10.1038/bjc.1988.15. PMC 2246686
 . PMID 3126791.
. PMID 3126791. - ↑ Porter, Pamela N.; Grishaver, Mary S.; Jones, Oliver W. (1974). "Characterization of human cystathionine β-synthase". Biochimica et Biophysica Acta. 364 (1): 128–39. doi:10.1016/0005-2744(74)90140-5. PMID 4433562.
- 1 2 3 Ogawa H, Gomi T, Konishi K, Date T, Nakashima H, Nose K, Matsuda Y, Peraino C, et al. (1989). "Human liver serine dehydratase. CDNA cloning and sequence homology with hydroxyamino acid dehydratases from other sources". Journal of Biological Chemistry. 264 (27): 15818–23. PMID 2674117.
- ↑ Christen, Philipp; Mehta, Perdeep K. (2001). "From cofactor to enzymes. The molecular evolution of pyridoxal-5′-phosphate-dependent enzymes". The Chemical Record. 1 (6): 436–47. doi:10.1002/tcr.10005. PMID 11933250.