Cav1.3
| View/Edit Human | View/Edit Mouse |
Calcium channel, voltage-dependent, L type, alpha 1D subunit (also known as Cav1.3) is a protein that in humans is encoded by the CACNA1D gene.[5] Cav1.3 channels belong to the Cav1 family, which form L-type calcium currents and are sensitive to selective inhibition by dihydropyridines (DHP).
Structure and function
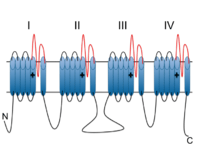
Voltage-dependent calcium channels (VDCC) are selectively permeable to calcium ions, mediating the movement of these ions in and out of excitable cells. At resting potential, these channels are closed, but when the membrane potential is depolarised these channels open. The influx of calcium ions into the cell can initiate a myriad of calcium-dependent processes including muscle contraction, gene expression, and secretion. Calcium-dependent processes can be halted by lowering intracellular calcium levels, which, for example, can be accomplished by calcium pumps.[6]
Voltage-dependent calcium channels are multi-proteins composed of α1, β, α2δ and γ subunits. The major subunit is α1, which forms the selectivity pore, voltage-sensor and gating apparatus of VDCCs. In Cav1.3 channels, the α1 subunit is α1D. This subunit differentiates Cav1.3 channels from other members of the Cav1 family, such as the predominant and better-studied Cav1.2, which has an α1C subunit. The significance of the α1 subunit also means that it is the primary target for calcium-channel blockers such as dihydropyridines. The remaining β, α2δ and γ subunits have auxiliary functions.
The α1 subunit has four homologous domains, each with six transmembrane segments. Within each homologous domain, the fourth transmembrane segment (S4) is positively charged, as opposed to the other five hydrophobic segments. This characteristic enables S4 to function as the voltage-sensor. Alpha-1D subunits belong to the Cav1 family, which is characterised by L-type calcium currents. Specifically, α1D subunits confer low-voltage activation and slowly inactivating Ca2+ currents, ideal for particular physiological functions such as neurotransmitter release in cochlea inner hair cells.
The biophysical properties of Cav1.3 channels are closely regulated by a C-terminal modulatory domain (CTM), which affects both the voltage dependence of activation and Ca2+ dependent inactivation.[7] Cav1.3 have a low affinity for DHP and activate at sub-threshold membrane potentials, making them ideal for a role in cardiac pacemaking.[8]
Regulation
Alternative splicing
Post-transcriptional alternative splicing of Cav1.3 is an extensive and vital regulatory mechanism. Alternative splicing can significantly affect the gating properties of the channel. Comparable to alternative splicing of Cav1.2 transcripts, which confers functional specificity,[9] it has recently been discovered that alternative splicing, particularly in the C-terminus, affects the pharmacological properties of Cav1.3. Strikingly, up to 8-fold differences in dihydropyridine sensitivity between alternatively spliced isoforms have been reported.[10]
Negative feedback
Cav1.3 channels are regulated by negative feedback to achieve Ca2+ homeostasis. Calcium ions are a critical second messenger, intrinsic to intracellular signal transduction. Extracellular calcium levels are approximated to be 12000-fold greater than intracellular levels. During calcium-dependent processes, the intracellular level of calcium rises by up to 100-fold. It is vitally important to regulate this calcium gradient, not least because high levels of calcium are toxic to the cell, and can induce apoptosis.
Ca2+-bound calmodulin (CaM) interacts with Cav1.3 to induce calcium-dependent inactivation (CDI). Recently, it has been shown that RNA editing of Cav1.3 transcripts is essential for CDI.[11] Contrary to expectation, RNA editing does not simply attenuate the binding of CaM, but weakens the pre-binding of Ca2+-free calmodulin (apoCaM) to channels. The upshot is that CDI is continuously tuneable by changes in levels of CaM.
Clinical significance
Hearing
Cav1.3 channels are widely expressed in humans. Notably, their expression predominates cochlea inner hair cells (IHCs). Cav1.3 have been shown through patch clamp experiments to be essential for normal IHC development and synaptic transmission.[12] Therefore, Cav1.3 are required for proper hearing.
Chromaffin cells
Cav1.3 are densely expressed in chromaffin cells. The low-voltage activation and slow inactivation of these channels makes them ideal for controlling excitability in these cells. Catecholamine secretion from chromaffin cells is particularly sensitive to L-type currents, associated with Cav1.3. Catecholamines have many systemic effects on multiple organs. In addition, L-type channels are responsible for exocytosis in these cells.[13]
Neurodegeneration
Parkinson's disease is the second most common neurodegenerative disease, in which the death of dopamine-producing cells in the substantia nigra of the midbrain leads to impaired motor function, perhaps best characterised by tremor. Recent evidence suggests that L-type Cav1.3 Ca2+ channels contribute to the death of dopaminergic neurones in patients with Parkinson's disease.[8] The basal activity of these neurones is highly dependent on L-type Ca2+ channels, such as Cav1.3, and it suggested that the pacemaking activity makes the dopaminergic neurones vulnerable to stressors that contribute to their death. Results suggest that inhibition of Cav1.3 is protective against the pathogenesis of Parkinson's.[8]
Inhibition of Cav1.3 can be achieved using blockers such as DHP. However, the broad action of DHP, which also affects Cav1.2, has limited therapeutic use as it would disturb a wide range of neurological processes to the detriment of the patient. In the face of this issue, a potent and highly selective Cav1.3 antagonist 1-(3-chlorophenethyl)-3-cyclopentylpyrimidine-2,4,6-(1H,3H,5H)-trione was recently identified and put forward as a candidate for the future treatment of Parkinson's.[14]
Prostate cancer
Recent evidence from immunostaining experiments shows that CACNA1D is highly expressed in prostate cancers compared with benign prostate tissues. Blocking L-type channels or knocking down gene expression of CACNA1D significantly suppressed cell-growth in prostate cancer cells.[15] It is important to recognise that this association does not represent a causal link between high levels of α1D protein and prostate cancer. Further investigation is needed to explore the role of CACNA1D gene overexpression in prostate cancer cell growth.
Aldosteronism
Mutations in the S6 segment of CACNA1D are associated with primary aldosteronism, which causes arterial hypertension. Alterations to the Gly403 residue result in channel activation at less depolarised potentials and impaired channel inactivation. This leads to increased Ca2+ influx, which in turn triggers aldosterone production.[16]
See also
References
- ↑ "Diseases that are genetically associated with CACNA1D view/edit references on wikidata".
- ↑ "Drugs that physically interact with Voltage-dependent L-type calcium channel subunit alpha-1D view/edit references on wikidata".
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ "Entrez Gene: CACNA1D calcium channel, voltage-dependent, L type, alpha 1D subunit".
- ↑ Brown BL, Walker SW, Tomlinson S (Aug 1985). "Calcium calmodulin and hormone secretion". Clinical Endocrinology. 23 (2): 201–18. doi:10.1111/j.1365-2265.1985.tb00216.x. PMID 2996810.
- ↑ Lieb A, Scharinger A, Sartori S, Sinnegger-Brauns MJ, Striessnig J (2012). "Structural determinants of CaV1.3 L-type calcium channel gating". Channels. 6 (3): 197–205. doi:10.4161/chan.21002. PMID 22760075.
- 1 2 3 Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ (Jun 2007). "'Rejuvenation' protects neurons in mouse models of Parkinson's disease". Nature. 447 (7148): 1081–6. doi:10.1038/nature05865. PMID 17558391.
- ↑ Liao P, Yu D, Lu S, Tang Z, Liang MC, Zeng S, Lin W, Soong TW (Nov 2004). "Smooth muscle-selective alternatively spliced exon generates functional variation in Cav1.2 calcium channels". The Journal of Biological Chemistry. 279 (48): 50329–35. doi:10.1074/jbc.m409436200. PMID 15381693.
- ↑ Huang H, Yu D, Soong TW (Oct 2013). "C-terminal alternative splicing of CaV1.3 channels distinctively modulates their dihydropyridine sensitivity". Molecular Pharmacology. 84 (4): 643–53. doi:10.1124/mol.113.087155. PMID 23924992.
- ↑ Bazzazi H, Ben Johny M, Adams PJ, Soong TW, Yue DT (Oct 2013). "Continuously tunable Ca(2+) regulation of RNA-edited CaV1.3 channels". Cell Reports. 5 (2): 367–77. doi:10.1016/j.celrep.2013.09.006. PMID 24120865.
- ↑ Brandt A, Striessnig J, Moser T (Nov 2003). "CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells". The Journal of Neuroscience. 23 (34): 10832–40. PMID 14645476.
- ↑ Vandael DH, Mahapatra S, Calorio C, Marcantoni A, Carbone E (Jul 2013). "Cav1.3 and Cav1.2 channels of adrenal chromaffin cells: emerging views on cAMP/cGMP-mediated phosphorylation and role in pacemaking". Biochimica et Biophysica Acta. 1828 (7): 1608–18. doi:10.1016/j.bbamem.2012.11.013. PMID 23159773.
- ↑ Kang S, Cooper G, Dunne SF, Dusel B, Luan CH, Surmeier DJ, Silverman RB (2012). "CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson's disease". Nature Communications. 3: 1146. doi:10.1038/ncomms2149. PMID 23093183.
- ↑ Chen R, Zeng X, Zhang R, Huang J, Kuang X, Yang J, Liu J, Tawfik O, Thrasher JB, Li B (Jul 2014). "Cav1.3 channel α1D protein is overexpressed and modulates androgen receptor transactivation in prostate cancers". Urologic Oncology. 32 (5): 524–36. doi:10.1016/j.urolonc.2013.05.011. PMID 24054868.
- ↑ Scholl UI, Goh G, Stölting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, Prasad ML, Hartung EA, Mauras N, Benson MR, Brady T, Shapiro JR, Loring E, Nelson-Williams C, Libutti SK, Mane S, Hellman P, Westin G, Åkerström G, Björklund P, Carling T, Fahlke C, Hidalgo P, Lifton RP (Sep 2013). "Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism". Nature Genetics. 45 (9): 1050–4. doi:10.1038/ng.2695. PMID 23913001.
Further reading
- Williams ME, Feldman DH, McCue AF, Brenner R, Velicelebi G, Ellis SB, Harpold MM (Jan 1992). "Structure and functional expression of alpha 1, alpha 2, and beta subunits of a novel human neuronal calcium channel subtype". Neuron. 8 (1): 71–84. doi:10.1016/0896-6273(92)90109-Q. PMID 1309651.
- Seino S, Chen L, Seino M, Blondel O, Takeda J, Johnson JH, Bell GI (Jan 1992). "Cloning of the alpha 1 subunit of a voltage-dependent calcium channel expressed in pancreatic beta cells". Proceedings of the National Academy of Sciences of the United States of America. 89 (2): 584–8. doi:10.1073/pnas.89.2.584. PMC 48283
 . PMID 1309948.
. PMID 1309948. - Seino S, Yamada Y, Espinosa R, Le Beau MM, Bell GI (Aug 1992). "Assignment of the gene encoding the alpha 1 subunit of the neuroendocrine/brain-type calcium channel (CACNL1A2) to human chromosome 3, band p14.3". Genomics. 13 (4): 1375–7. doi:10.1016/0888-7543(92)90078-7. PMID 1324226.
- Chin HM, Kozak CA, Kim HL, Mock B, McBride OW (Dec 1991). "A brain L-type calcium channel alpha 1 subunit gene (CCHL1A2) maps to mouse chromosome 14 and human chromosome 3". Genomics. 11 (4): 914–9. doi:10.1016/0888-7543(91)90014-6. PMID 1664412.
- Mori Y, Friedrich T, Kim MS, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T (Apr 1991). "Primary structure and functional expression from complementary DNA of a brain calcium channel". Nature. 350 (6317): 398–402. doi:10.1038/350398a0. PMID 1849233.
- Yamada Y, Masuda K, Li Q, Ihara Y, Kubota A, Miura T, Nakamura K, Fujii Y, Seino S, Seino Y (May 1995). "The structures of the human calcium channel alpha 1 subunit (CACNL1A2) and beta subunit (CACNLB3) genes". Genomics. 27 (2): 312–9. doi:10.1006/geno.1995.1048. PMID 7557998.
- Puro DG, Hwang JJ, Kwon OJ, Chin H (Apr 1996). "Characterization of an L-type calcium channel expressed by human retinal Müller (glial) cells". Brain Research. Molecular Brain Research. 37 (1-2): 41–8. doi:10.1016/0169-328X(96)80478-5. PMID 8738134.
- Yang SN, Larsson O, Bränström R, Bertorello AM, Leibiger B, Leibiger IB, Moede T, Köhler M, Meister B, Berggren PO (Aug 1999). "Syntaxin 1 interacts with the L(D) subtype of voltage-gated Ca(2+) channels in pancreatic beta cells". Proceedings of the National Academy of Sciences of the United States of America. 96 (18): 10164–9. doi:10.1073/pnas.96.18.10164. PMC 17860
 . PMID 10468580.
. PMID 10468580. - Bell DC, Butcher AJ, Berrow NS, Page KM, Brust PF, Nesterova A, Stauderman KA, Seabrook GR, Nürnberg B, Dolphin AC (Feb 2001). "Biophysical properties, pharmacology, and modulation of human, neuronal L-type (alpha(1D), Ca(V)1.3) voltage-dependent calcium currents". Journal of Neurophysiology. 85 (2): 816–27. PMID 11160515.
- Rosenthal R, Thieme H, Strauss O (Apr 2001). "Fibroblast growth factor receptor 2 (FGFR2) in brain neurons and retinal pigment epithelial cells act via stimulation of neuroendocrine L-type channels (Ca(v)1.3)". FASEB Journal. 15 (6): 970–7. doi:10.1096/fj.00-0188com. PMID 11292657.
- Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW (Jul 2001). "A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2". Science. 293 (5527): 98–101. doi:10.1126/science.293.5527.98. PMID 11441182.
- Namkung Y, Skrypnyk N, Jeong MJ, Lee T, Lee MS, Kim HL, Chin H, Suh PG, Kim SS, Shin HS (Oct 2001). "Requirement for the L-type Ca(2+) channel alpha(1D) subunit in postnatal pancreatic beta cell generation". The Journal of Clinical Investigation. 108 (7): 1015–22. doi:10.1172/JCI13310. PMC 200955
 . PMID 11581302.
. PMID 11581302. - Stokes L, Gordon J, Grafton G (May 2004). "Non-voltage-gated L-type Ca2+ channels in human T cells: pharmacology and molecular characterization of the major alpha pore-forming and auxiliary beta-subunits". The Journal of Biological Chemistry. 279 (19): 19566–73. doi:10.1074/jbc.M401481200. PMID 14981074.
- Qu Y, Baroudi G, Yue Y, Boutjdir M (Jun 2005). "Novel molecular mechanism involving alpha1D (Cav1.3) L-type calcium channel in autoimmune-associated sinus bradycardia". Circulation. 111 (23): 3034–41. doi:10.1161/CIRCULATIONAHA.104.517326. PMID 15939813.
- Baroudi G, Qu Y, Ramadan O, Chahine M, Boutjdir M (Oct 2006). "Protein kinase C activation inhibits Cav1.3 calcium channel at NH2-terminal serine 81 phosphorylation site". American Journal of Physiology. Heart and Circulatory Physiology. 291 (4): H1614–22. doi:10.1152/ajpheart.00095.2006. PMID 16973824.
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (Nov 2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell. 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983.
External links
- CACNA1D protein, human at the US National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.