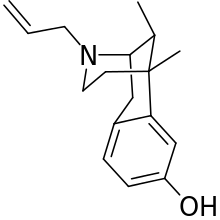
Alazocine
 | |
| Clinical data | |
|---|---|
| Routes of administration | ? |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
14198-28-8 |
| PubChem (CID) | 3036246 |
| ChemSpider |
2300306 |
| ChEMBL |
CHEMBL330376 |
| Chemical and physical data | |
| Formula | C17H23NO |
| Molar mass | 257.37 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Alazocine ((-)-SKF-10,047), or (-)-N-allylnormetazocine ((-)-ANMC), was the first drug discovered to act as a σ1 receptor agonist (Ki = 24 nM).[1][2][3] It has no significant affinity for the σ2 receptor.[3] Alazocine also acts as a κ-opioid receptor partial agonist (Ki = 0.4 nM; EC50 = 24 nM; Emax = 66%),[4] and to a much lesser extent, as an NMDA receptor antagonist (Ki = 587 nM).[3][5]
See also
References
- ↑ Iwamoto ET (February 1981). "Pharmacologic effects of N-allylnormetazocine (SKF-10047)". NIDA Research Monograph. 34: 82–8. PMID 6783955.
- ↑ Shearman GT, Herz A (August 1982). "Non-opioid psychotomimetic-like discriminative stimulus properties of N-allylnormetazocine (SKF 10,047) in the rat". European Journal of Pharmacology. 82 (3–4): 167–72. doi:10.1016/0014-2999(82)90506-4. PMID 6290235.
- 1 2 3 Chou YC, Liao JF, Chang WY, Lin MF, Chen CF (March 1999). "Binding of dimemorfan to sigma-1 receptor and its anticonvulsant and locomotor effects in mice, compared with dextromethorphan and dextrorphan". Brain Research. 821 (2): 516–9. doi:10.1016/S0006-8993(99)01125-7. PMID 10064839.
- ↑ Gharagozlou, Parham; Hashemi, Ezzat; DeLorey, TimothyM; Clark, J David; Lameh, Jelveh (2006). "Pharmacological profiles of opioid ligands at Kappa opioid receptors". BMC Pharmacology. 6 (1): 3. doi:10.1186/1471-2210-6-3. ISSN 1471-2210.
- ↑ Gharagozlou P, Hashemi E, DeLorey TM, Clark JD, Lameh J (2006). "Pharmacological profiles of opioid ligands at Kappa opioid receptors". BMC Pharmacology. 6 (1): 3. doi:10.1186/1471-2210-6-3. PMC 1403760
 . PMID 16433932.
. PMID 16433932.
| Receptor (ligands) |
| ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (blockers) |
| ||||||||||||||||||||||||||||||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||
See also: GABAergics • GHBergics • Glycinergics | |||||||||||||||||||||||||||||||||||||||||||
| MOR |
|
|---|---|
| DOR |
|
| KOR |
|
| NOP |
|
| Unsorted |
|
| Others |
|
See also: Peptide receptor modulators | |
| Agonists |
|
|---|---|
| Antagonists |
|
| Unknown / unsorted |
|
This article is issued from Wikipedia - version of the 5/22/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.