Naphthalene
| |||
 | |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Naphthalene[1] | |||
| Systematic IUPAC name
Bicyclo[4.4.0]deca-1,3,5,7,9-pentaene Bicyclo[4.4.0]deca-2,4,6,8,10-pentaene | |||
| Other names
white tar, camphor tar, tar camphor, naphthalin, naphthaline, antimite, albocarbon, hexalene, mothballs, moth flakes | |||
| Identifiers | |||
| 91-20-3 | |||
| 3D model (Jmol) | Interactive image | ||
| 1421310 | |||
| ChEBI | CHEBI:16482 | ||
| ChEMBL | ChEMBL16293 | ||
| ChemSpider | 906 | ||
| ECHA InfoCard | 100.001.863 | ||
| EC Number | 214-552-7 | ||
| 3347 | |||
| KEGG | C00829 | ||
| PubChem | 931 | ||
| RTECS number | QJ0525000 | ||
| UNII | 2166IN72UN | ||
| |||
| |||
| Properties | |||
| C10H8 | |||
| Molar mass | 128.17 g·mol−1 | ||
| Appearance | White solid crystals/ flakes | ||
| Odor | Strong odor of coal tar | ||
| Density | 1.145 g/cm3 (15.5 °C)[2] 1.0253 g/cm3 (20 °C)[3] 0.9625 g/cm3 (100 °C)[2] | ||
| Melting point | 78.2 °C (172.8 °F; 351.3 K) 80.26 °C (176.47 °F; 353.41 K) at 760 mmHg[3] | ||
| Boiling point | 217.97 °C (424.35 °F; 491.12 K) at 760 mmHg[2][3] | ||
| 19 mg/L (10 °C) 31.6 mg/L (25 °C) 43.9 mg/L (34.5 °C) 80.9 mg/L (50 °C)[3] 238.1 mg/L (73.4 °C)[4] | |||
| Solubility | Soluble in alcohols, liquid ammonia, carboxylic acids, C6H6, SO2,[4] CCl4, CS2, toluene, aniline[5] | ||
| Solubility in ethanol | 5 g/100 g (0 °C) 11.3 g/100 g (25 °C) 19.5 g/100 g (40 °C) 179 g/100 g (70 °C)[5] | ||
| Solubility in acetic acid | 6.8 g/100 g (6.75 °C) 13.1 g/100 g (21.5 °C) 31.1 g/100 g (42.5 °C) 111 g/100 g (60 °C)[5] | ||
| Solubility in chloroform | 19.5 g/100 g (0 °C) 35.5 g/100 g (25 °C) 49.5 g/100 g (40 °C) 87.2 g/100 g (70 °C)[5] | ||
| Solubility in hexane | 5.5 g/100 g (0 °C) 17.5 g/100 g (25 °C) 30.8 g/100 g (40 °C) 78.8 g/100 g (70 °C)[5] | ||
| Solubility in butyric acid | 13.6 g/100 g (6.75 °C) 22.1 g/100 g (21.5 °C) 131.6 g/100 g (60 °C)[5] | ||
| log P | 3.34[3] | ||
| Vapor pressure | 8.64 Pa (20 °C) 23.6 Pa (30 °C) 0.93 kPa (80 °C)[4] 2.5 kPa (100 °C)[6] | ||
| Henry's law constant (kH) |
0.42438 L·atm/mol[3] | ||
| Thermal conductivity | 98 kPa: 0.1219 W/m·K (372.22 K) 0.1174 W/m·K (400.22 K) 0.1152 W/m·K (418.37 K) 0.1052 W/m·K (479.72 K)[7] | ||
| Refractive index (nD) |
1.5898[3] | ||
| Viscosity | 0.964 cP (80 °C) 0.761 cP (100 °C) 0.217 cP (150 °C)[8] | ||
| Structure | |||
| Monoclinic[9] | |||
| P21/b[9] | |||
| C5 2h[9] | |||
| α = 90°, β = 122.92°, γ = 90° | |||
| Thermochemistry | |||
| 165.72 J/mol·K[3] | |||
| Std molar entropy (S |
167.39 J/mol·K[3][6] | ||
| Std enthalpy of formation (ΔfH |
78.53 kJ/mol[3] | ||
| Gibbs free energy (ΔfG˚) |
201.585 kJ/mol[3] | ||
| Std enthalpy of combustion (ΔcH |
-5156.3 kJ/mol[3] | ||
| Hazards | |||
| Main hazards | Flammable, sensitizer, possible carcinogen. Dust can form explosive mixtures with air | ||
| GHS pictograms |     [10] [10] | ||
| GHS signal word | Danger | ||
| H228, H302, H351, H410[10] | |||
| P210, P273, P281, P501[10] | |||
| EU classification (DSD) |
| ||
| R-phrases | R22, R40, R50/53 | ||
| S-phrases | (S2), S36/37, S46, S60, S61 | ||
| NFPA 704 | |||
| Flash point | 80 °C (176 °F; 353 K)[10] | ||
| 525 °C (977 °F; 798 K)[10] | |||
| Explosive limits | 5.9%[10] | ||
| 10 ppm[3] (TWA), 15 ppm[3] (STEL) | |||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) |
1800 mg/kg (rat, oral) 490 mg/kg (rat, oral) 1200 mg/kg (guinea pig, oral) 533 mg/kg (mouse, oral)[11] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 10 ppm (50 mg/m3)[12] | ||
| REL (Recommended) |
TWA 10 ppm (50 mg/m3) ST 15 ppm (75 mg/m3)[12] | ||
| IDLH (Immediate danger) |
250 ppm[12] | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Naphthalene is an organic compound with formula C
10H
8. It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass.[13] As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is best known as the main ingredient of traditional mothballs.
History
In the early 1820s, two separate reports described a white solid with a pungent odor derived from the distillation of coal tar. In 1821, John Kidd cited these two disclosures and then described many of this substance's properties and the means of its production. He proposed the name naphthaline, as it had been derived from a kind of naphtha (a broad term encompassing any volatile, flammable liquid hydrocarbon mixture, including coal tar).[14] Naphthalene's chemical formula was determined by Michael Faraday in 1826. The structure of two fused benzene rings was proposed by Emil Erlenmeyer in 1866,[15] and confirmed by Carl Gräbe three years later.[16]
Structure and reactivity
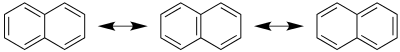
A naphthalene molecule can be viewed as the fusion of a pair of benzene rings. (In organic chemistry, rings are fused if they share two or more atoms.) As such, naphthalene is classified as a benzenoid polycyclic aromatic hydrocarbon (PAH). There are two sets of equivalent hydrogen atoms: the alpha positions are numbered 1, 4, 5, and 8 (per diagram in right margin), and the beta positions, 2, 3, 6, and 7.
Unlike benzene, the carbon–carbon bonds in naphthalene are not of the same length. The bonds C1−C2, C3−C4, C5−C6 and C7−C8 are about 1.37 Å (137 pm) in length, whereas the other carbon–carbon bonds are about 1.42 Å (142 pm) long. This difference, established by X-ray diffraction,[17] is consistent with the valence bond model in naphthalene and in particular, with the theorem of cross-conjugation. This theorem would describe naphthalene as an aromatic benzene unit bonded to a diene but not extensively conjugated to it (at least in the ground state). As such, naphthalene possesses several resonance structures.
Two isomers are possible for mono-substituted naphthalenes, corresponding to substitution at an alpha or beta position. Bicyclo[6.2.0]decapentaene is a structural isomer with a fused 4–8 ring system.[18]
Reactions with electrophiles
In electrophilic aromatic substitution reactions, naphthalene reacts more readily than benzene. For example, chlorination and bromination of naphthalene proceeds without a catalyst to give 1-chloronaphthalene and 1-bromonaphthalene. Likewise, whereas both benzene and naphthalene can be alkylated using Friedel–Crafts reactions, naphthalene can also be easily alkylated by reaction with alkenes or alcohols, using sulfuric or phosphoric acid catalysts.
In terms of regiochemistry, electrophiles attack occurs at the alpha position. The selectivity for alpha over beta substitution can be rationalized in terms of the resonance structures of the intermediate: for the alpha substitution intermediate, seven resonance structures can be drawn, of which four preserve an aromatic ring. For beta substitution, the intermediate has only six resonance structures, and only two of these are aromatic. Sulfonation, however, gives a mixture of the "alpha" product 1-naphthalenesulfonic acid and the "beta" product 2-naphthalenesulfonic acid, with the ratio dependent on reaction conditions. The 1-isomer forms predominantly at 25 °C, and the 2-isomer at 160 °C. Sulfonation to give the 1- and 2-sulfonic acid occurs readily:
- H
2SO
4 + C
10H
8 → C
10H
7−SO
3H + H
2O
Further sulfonation occurs to give di-, tri-, and tetrasulfonic acids.
Lithiation
Analogous to the synthesis of phenyllithium is the conversion of 1-bromonaphthalene to 1-lithionaphthalene, a lithium-halogen exchange:
- C10H7Br + BuLi → C10H7Li + BuBr
The resulting lithionaphthalene undergoes a second lithiation, in contrast to the behavior of phenyllithium. These 1,8-dilithio derivatives are precursors to a host of peri-naphthalene derivatives.[19]
Reduction and oxidation
With alkali metals, naphthalene forms the dark blue-green radical anion salts such as sodium naphthalenide, Na+C10H−
8. The naphthalenide salts are strong reducing agents.
Naphthalene can be hydrogenated under high pressure in the presence of metal catalysts to give 1,2,3,4-tetrahydronaphthalene(C
10H
12), also known as tetralin. Further hydrogenation yields decahydronaphthalene or decalin (C
10H
18).
Oxidation with O
2 in the presence of a vanadium catalyst gives phthalic anhydride:
- C10H8 + 4.5 O2 → C6H4(CO)2O + 2 CO2 + 2 H2O
This reaction is the basis of the main use of naphthalene. Oxidation can also be effected using conventional stoichiometric chromate or permanganate reagents.
Production
Most naphthalene is derived from coal tar. From the 1960s until the 1990s, significant amounts of naphthalene were also produced from heavy petroleum fractions during petroleum refining, but today petroleum-derived naphthalene represents only a minor component of naphthalene production.
Naphthalene is the most abundant single component of coal tar. Although the composition of coal tar varies with the coal from which it is produced, typical coal tar is about 10% naphthalene by weight. In industrial practice, distillation of coal tar yields an oil containing about 50% naphthalene, along with twelve other aromatic compounds. This oil, after being washed with aqueous sodium hydroxide to remove acidic components (chiefly various phenols), and with sulfuric acid to remove basic components, undergoes fractional distillation to isolate naphthalene. The crude naphthalene resulting from this process is about 95% naphthalene by weight. The chief impurities are the sulfur-containing aromatic compound benzothiophene (< 2%), indane (0.2%), indene (< 2%), and methylnaphthalene (< 2%). Petroleum-derived naphthalene is usually purer than that derived from coal tar. Where required, crude naphthalene can be further purified by recrystallization from any of a variety of solvents, resulting in 99% naphthalene by weight, referred to as 80 °C (melting point). Approximately 1.3M tons are produced annually.[20]
In North America, the coal tar producers are Koppers Inc., Ruetgers Canada Inc. and Recochem Inc., and the primary petroleum producer is Monument Chemical Inc. In Western Europe the well-known producers are Koppers, Ruetgers, and Deza. In Eastern Europe, naphthalene is produced by a variety of integrated metallurgy complexes (Severstal, Evraz, Mechel, MMK) in Russia, dedicated naphthalene and phenol makers INKOR and Yenakievsky Metallurgy plant in Ukraine, and ArcelorMittal Temirtau in Kazakhstan.
Other sources and occurrences
Aside from coal tar, trace amounts of naphthalene are produced by magnolias and certain species of deer, as well as the Formosan subterranean termite, possibly produced by the termite as a repellant against "ants, poisonous fungi and nematode worms."[21] Some strains of the endophytic fungus Muscodor albus produce naphthalene among a range of volatile organic compounds, while Muscodor vitigenus produces naphthalene almost exclusively.[22]
Naphthalene has been found in meteorites: n C
10H
7−SO
3H + n CH
2=O → SO
3H−C
10H
7−(−CH
2−C
10H
7−SO
3H)
n + n H
2O
- Neutralization step (naphthalene sulfonic acid condensate plus sodium hydroxide):
Naphthalene in the interstellar medium
Naphthalene has been tentatively detected in the interstellar medium in the direction of the star Cernis 52 in the constellation Perseus.[23][24] More than 20% of the carbon in the universe may be associated with polyaromatic hydrocarbons, including naphthalene.[25]
Protonated cations of naphthalene (C
10H+
9) are the source of part of the spectrum of the Unidentified Infrared Emissions (UIRs). Protonated naphthalene differs from neutral naphthalene (e.g. that used in mothballs) in that it has an additional hydrogen atom. The UIRs from
"naphthalene cation" (C
10H+
8) have been observed by astronomers. This research has been publicized as "mothballs in space."[26]
Uses
Naphthalene is used mainly as a precursor to other chemicals. The single largest use of naphthalene is the industrial production of phthalic anhydride, although more phthalic anhydride is made from o-xylene. Many azo dyes are produced from naphthalene. The insecticide 1-naphthyl-N-methylcarbamate (carbaryl). Other useful agrichemicals include naphthoxyacetic acids.
.
Naphthalenesulfonic acids and sulfonates
Many naphthalenesulfonic acids and sulfonates are useful. Alkyl naphthalene sulfonate are surfactants, The aminonaphthalenesulfonic acids, naphthalenes substituted with amines and sulfonic acids, are intermediates in the preparation of many synthetic dyes. The hydrogenated naphthalenes tetrahydronaphthalene (tetralin) and decahydronaphthalene (decalin) are used as low-volatility solvents. Naphthalene sulfonic acids are also used in the synthesis of 1-naphthol and 2-naphthol, precursors for various dyestuffs, pigments, rubber processing chemicals and other chemicals and pharmaceuticals.[20]
Naphthalene sulfonic acids are used in the manufacture of naphthalene sulfonate polymer plasticizers (dispersants), which are used to produce concrete and plasterboard (wallboard or drywall). They are also used as dispersants in synthetic and natural rubbers, and as tanning agents (syntans) in leather industries, agricultural formulations (dispersants for pesticides), dyes and as a dispersant in lead–acid battery plates.
Naphthalene sulfonate polymers are produced by treating naphthalenesulfonic acid with formaldehyde, followed by neutralization with sodium hydroxide or calcium hydroxide. These products are commercially sold in solution (water) or dry powder form.
Niche applications
Being inexpensive, naphthalene finds many niche uses.
Laboratory uses
Molten naphthalene provides an excellent solubilizing medium for poorly soluble aromatic compounds. In many cases it is more efficient than other high-boiling solvents, such as dichlorobenzene, benzonitrile, nitrobenzene and durene. The reaction of C60 with anthracene is conveniently conducted in refluxing naphthalene to give the 1:1 Diels–Alder adduct.[27] The aromatization of hydroporphyrins has been achieved using a solution of DDQ in naphthalene.[28]
Wetting agent and surfactant
Alkyl naphthalene sulfonates (ANS) are used in many industrial applications as nondetergent wetting agents that effectively disperse colloidal systems in aqueous media. The major commercial applications are in the agricultural chemical industry, which uses ANS for wettable powder and wettable granular (dry-flowable) formulations, and the textile and fabric industry, which utilizes the wetting and defoaming properties of ANS for bleaching and dyeing operations.
As a fumigant
Naphthalene has been used as a household fumigant. It was once the primary ingredient in mothballs, although its use has largely been replaced in favor of alternatives such as 1,4-dichlorobenzene. In a sealed container containing naphthalene pellets, naphthalene vapors build up to levels toxic to both the adult and larval forms of many moths that attack textiles. Other fumigant uses of naphthalene include use in soil as a fumigant pesticide, in attic spaces to repel animals and insects, and in museum storage-drawers and cupboards to protect the contents from attack by insect pests.
Naphthalene is a repellent to opossums.[29][30]
Pyrotechnics
It is used in pyrotechnic special effects such as the generation of black smoke and simulated explosions. It is used to create artificial pores in the manufacture of high-porosity grinding wheels. In the past, naphthalene was administered orally to kill parasitic worms in livestock. Naphthalene and its alkyl homologs are the major constituents of creosote. Naphthalene is used in engineering to study heat transfer using mass sublimation.
Health effects
Exposure to large amounts of naphthalene may damage or destroy red blood cells, most commonly in people with an underlying G6PD (glucose-6-phosphate dehydrogenase) deficiency.[31] Over 400 million people have an inherited condition called glucose-6-phosphate dehydrogenase deficiency. Humans, in particular children, have developed this condition, known as hemolytic anemia, after ingesting mothballs or deodorant blocks containing naphthalene. Symptoms include fatigue, lack of appetite, restlessness, and pale skin. Exposure to large amounts of naphthalene may cause confusion, nausea, vomiting, diarrhea, blood in the urine, and jaundice (yellow coloration of the skin due to dysfunction of the liver).[32]
When the US National Toxicology Program (NTP) exposed male and female rats and mice to naphthalene vapors on weekdays for two years,[33] male and female rats exhibited evidence of carcinogenesis with increased incidences of adenoma and neuroblastoma of the nose, female mice exhibited some evidence of carcinogenesis based on increased incidences of alveolar and bronchiolar adenomas of the lung, and male mice exhibited no evidence of carcinogenesis.
The International Agency for Research on Cancer (IARC)[34] classifies naphthalene as possibly carcinogenic to humans and animals (Group 2B). The IARC also points out that acute exposure causes cataracts in humans, rats, rabbits, and mice; and that hemolytic anemia (described above) can occur in children and infants after oral or inhalation exposure or after maternal exposure during pregnancy. Under California's Proposition 65, naphthalene is listed as "known to the State to cause cancer".[35] A probable mechanism for the carcinogenic effects of mothballs and some types of air fresheners containing naphthalene has been identified.[36][37]
Regulation
US government agencies have set occupational exposure limits to naphthalene exposure. The Occupational Safety and Health Administration has set a permissible exposure limit at 10 ppm (50 mg/m3) over an eight-hour time-weighted average. The National Institute for Occupational Safety and Health has set a recommended exposure limit at 10 ppm (50 mg/m3) over an eight-hour time-weighted average, as well as a short-term exposure limit at 15 ppm (75 mg/m3).[38]
Mothballs and other products containing naphthalene have been banned within the EU since 2008.[39][40]
In China, the use of naphthalene in mothballs is forbidden.[41] Danger to human health and the common use of natural camphor are cited as reasons for the ban.
See also
- Camphor
- Dialin, Tetralin, Decalin
- List of interstellar and circumstellar molecules
- Mothballs
- 1-Naphthol, 2-Naphthol
- Sodium naphthalenide
- Wagner-Jauregg reaction (classic naphthalene synthesis)
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 13, 35, 204, 207, 221–222, 302, 457, 461, 469, 601, 650. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- 1 2 3 "Ambient Water Quality Criteria for Naphthalene" (PDF). United States Environmental Protection Agency. Retrieved 2014-06-21.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- 1 2 3 Anatolievich, Kiper Ruslan. "naphthalene". chemister.ru. Retrieved 2014-06-21.
- 1 2 3 4 5 6 Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). New York: D. Van Nostrand Company. pp. 443–446.
- 1 2 Naphthalene in Linstrom, P.J.; Mallard, W.G. (eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology, Gaithersburg MD. http://webbook.nist.gov (retrieved 2014-05-24)
- ↑ "Thermal Conductivity of Naphthalene". DDBST GmbH. DDBST GmbH. Retrieved 2014-06-21.
- ↑ "Dynamic Viscosity of Naphthalene". DDBST GmbH. DDBST GmbH. Retrieved 2014-06-21.
- 1 2 3 4 Douglas, Bodie E.; Ho, Shih-Ming (2007). Structure and Chemistry of Crystalline Solids. New York: Springer Science+Business Media, Inc. p. 288. ISBN 0-387-26147-8.
- 1 2 3 4 5 6 Sigma-Aldrich Co., Naphthalene. Retrieved on 2014-06-21.
- ↑ "Naphthalene". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 "NIOSH Pocket Guide to Chemical Hazards #0439". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Amoore JE, Hautala E (1983). "Odor as an aid to chemical safety: Odor thresholds compared with threshold limit values and volatiles for 214 industrial chemicals in air and water dilution". J Appl Toxicology. 3 (6): 272–290. doi:10.1002/jat.2550030603.
- ↑ John Kidd (1821). "Observations on Naphthalene, a peculiar substance resembling a concrete essential oil, which is produced during the decomposition of coal tar, by exposure to a red heat". Philosophical Transactions. 111: 209–221. doi:10.1098/rstl.1821.0017.
- ↑ Emil Erlenmeyer (1866). "Studien über die s. g. aromatischen Säuren". Annalen der Chemie und Pharmacie. 137 (3): 327–359. doi:10.1002/jlac.18661370309.
- ↑ C. Graebe (1869) "Ueber die Constitution des Naphthalins" (On the structure of naphthalene), Annalen der Chemie und Pharmacie, 149 : 20–28.
- ↑ Cruickshank, D. W. J.; Sparks, R. A. (18 October 1960). "Experimental and Theoretical Determinations of Bond Lengths in Naphthalene, Anthracene and Other Hydrocarbons". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 258 (1293): 270–285. doi:10.1098/rspa.1960.0187.
- ↑ Dieter Cremer; Thomas Schmidt; Charles W. Bock (1985). "Theoretical determination of molecular structure and conformation. 14. Is bicyclo[6.2.0]decapentaene aromatic or antiaromatic?". J. Org. Chem. 50 (15): 2684–2688. doi:10.1021/jo00215a018.
- ↑ van Soolingen, J.; de Lang, R. J.; den Besten, R.; Klusener, P. A. A.; Veldman, N.; Spek, A. L.; Brandsma, L., "A simple procedure for the preparation of 1,8-bis(diphenylphosphino)naphthalene", Synthetic Communications 1995, 25, 1741-1744.
- 1 2 Gerd Collin, Hartmut Höke, Helmut Greim "Naphthalene and Hydronaphthalenes" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2003. doi:10.1002/14356007.a17_001.pub2. Article Online Posting Date: March 15, 2003.
- ↑ "Termite 'mothball' keep insects at bay". Sci/Tech. BBC News. April 8, 1998.
- ↑ Daisy BH, Strobel GA, Castillo U, et al. (November 2002). "Naphthalene, an insect repellent, is produced by Muscodor vitigenus, a novel endophytic fungus". Microbiology (Reading, Engl.). 148 (Pt 11): 3737–41. doi:10.1099/00221287-148-11-3737. PMID 12427963.
- ↑ "Interstellar Space Molecules That Help Form Basic Life Structures Identified". Science Daily. September 2008.
- ↑ Iglesias-Groth, S.; et al. (2008-09-20), "Evidence for the Naphthalene Cation in a Region of the Interstellar Medium with Anomalous Microwave Emission", The Astrophysical Journal Letters, 685: L55–L58, arXiv:0809.0778
 , Bibcode:2008ApJ...685L..55I, doi:10.1086/592349 - This spectral assignment has not been independently confirmed, and is described by the authors as "tentative" (page L58).
, Bibcode:2008ApJ...685L..55I, doi:10.1086/592349 - This spectral assignment has not been independently confirmed, and is described by the authors as "tentative" (page L58). - ↑ Hoover, Rachel (February 21, 2014). "Need to Track Organic Nano-Particles Across the Universe? NASA's Got an App for That". NASA. Retrieved February 22, 2014.
- ↑ "Mothballs in Space". Astrobiology Magazine. Retrieved December 25, 2008.
- ↑ K. Komatsua; Y. Murataa; N. Sugitaa; K. Takeuchib; T.S.M. Wan (1993). "Use of naphthalene as a solvent for selective formation of the 1:1 Diels–Alder adduct of C60 with anthracene". Tetrahedron Letters. 34 (52): 8473–8476. doi:10.1016/S0040-4039(00)61362-X.
- ↑ M.A. Filatov; A.V. Cheprakov (2011). "The synthesis of new tetrabenzo- and tetranaphthoporphyrins via the addition reactions of 4,7-dihydroisoindole". Tetrahedron. 67 (19): 3559–3566. doi:10.1016/j.tet.2011.01.052.
- ↑ "Summary of Possum Repellent Study". Archived from the original on September 28, 2013.
- ↑ "Removing a possums from your roof", NSW Department of the Environment and Heritage, http://www.environment.nsw.gov.au/animals/RemovingAPossumFromYourRoof.htm
- ↑ Santucci K, Shah B (Jan 2000). "Association of naphthalene with acute hemolytic anemia". Acad Emerg Med. 7 (1): 42–7. PMID 10894241.
- ↑ MedlinePlus Encyclopedia Naphthalene poisoning
- ↑ "NTP Technical Reports 410 and 500". NTP Technical Reports 410 and 500, available from NTP: Long-Term Abstracts & Reports. Archived from the original on October 24, 2004. Retrieved March 6, 2005.
- ↑ "IARC Monographs on the Evaluation of Carcinogenic Risks to Humans". Monographs on the Evaluation of Carcinogenic Risks to Humans, Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene, Vol. 82 (2002) (p. 367). Retrieved December 25, 2008.
- ↑ Proposition 65, Office of Environmental Health Hazard Assessment
- ↑ "Scientists May Have Solved Mystery Of Carcinogenic Mothballs", Physorg.com, June 20, 2006.
- ↑ "Mothballs, air fresheners and cancer". Environmental Health Association of Nova Scotia. Environmental Health Association of Nova Scotia. Retrieved 24 May 2013.
- ↑ CDC - NIOSH Pocket Guide to Chemical Hazards
- ↑ Alderson, Andrew (15 Nov 2008). "Holy straight bananas – now the Eurocrats are banning moth balls". The Telegraph. Retrieved 2013-11-23.
- ↑ Gray, Kerrina (17 November 2013). "Council warned against use of poisonous moth balls". Your Local Guardian. Newsquest (London) Ltd. Retrieved 2012-11-23.
- ↑ 国务院经贸办、卫生部关于停止生产和销售萘丸提倡使用樟脑制品的通知(国经贸调(1993)64号)
External links
| Wikimedia Commons has media related to Naphthalene. |
- Naphthalene—National Pesticide Information Center
- Naphthalene—EPA Air Toxics Web Site
- Naphthalene (PIM 363)—mostly on toxicity of naphthalene
- Naphthalene—CDC – NIOSH Pocket Guide to Chemical Hazards
- Naphthalene in the Pesticide Properties DataBase (PPDB)