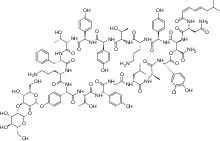
Ramoplanin
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number |
76168-82-6 |
| PubChem (CID) | 16132338 |
| UNII |
0WX9996O2G |
| ChEMBL |
CHEMBL1095892 |
| ECHA InfoCard | 100.161.388 |
| Chemical and physical data | |
| Formula | C119H154ClN21O40 |
| Molar mass | 2554.07 g/mol |
| | |
Ramoplanin (INN) is a glycolipodepsipeptide antibiotic drug derived from strain ATCC 33076 of Actinoplanes.[1]
Mechanism
It exerts its bacteriocidal effect by inhibiting cell wall biosynthesis, acting by inhibiting the transglycosylation step of peptidoglycan synthesis.[2]
Uses
Its development has been fast-tracked by the U.S. Food and Drug Administration as a treatment for multiple antibiotic-resistant Clostridium difficile infection of the gastrointestinal tract,[3] although it is unstable in the bloodstream, so can be taken only orally against such infections.[4][5][6]
References
- ↑ Farver DK, Hedge DD, Lee SC. Ramoplanin: a lipoglycodepsipeptide antibiotic. Annals of Pharmacotherapy. 2005 May;39(5):863-8. PMID 15784805
- ↑ Fang X, Tiyanont K, Zhang Y, Wanner J, Boger D, Walker S. The mechanism of action of ramoplanin and enduracidin. Molecular Biosystems. 2006 Jan;2(1):69-76. PMID 16880924
- ↑ Fulco P, Wenzel RP. Ramoplanin: a topical lipoglycodepsipeptide antibacterial agent. Expert Review of Anti Infective Therapy. 2006 Dec;4(6):939-45. PMID 17181409
- ↑ Scheinfeld N. A comparison of available and investigational antibiotics for complicated skin infections and treatment-resistant Staphylococcus aureus and enterococcus. Journal of Drugs in Dermatology. 2007 Jan;6(1):97-103. PMID 17373167
- ↑ Balagopal A, Sears CL. Clostridium difficile: new therapeutic options. Current Opinion in Pharmacology. 2007 Oct;7(5):455-8. PMID 17644040
- ↑ Gerding DN, Muto CA, Owens RC Jr. Treatment of Clostridium difficile infection. Clinical Infectious Diseases. 2008 Jan 15;46 Suppl 1:S32-42. PMID 18177219
This article is issued from Wikipedia - version of the 2/16/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.